Neuroprotection resulting from insufficiency of RANBP2 is associated with the modulation of protein and lipid homeostasis of functionally diverse but linked pathways in response to oxidative stress
- PMID: 20682751
- PMCID: PMC2931537
- DOI: 10.1242/dmm.004648
Neuroprotection resulting from insufficiency of RANBP2 is associated with the modulation of protein and lipid homeostasis of functionally diverse but linked pathways in response to oxidative stress
Abstract
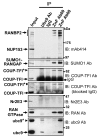
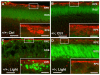
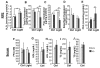
Oxidative stress is a deleterious stressor associated with a plethora of disease and aging manifestations, including neurodegenerative disorders, yet very few factors and mechanisms promoting the neuroprotection of photoreceptor and other neurons against oxidative stress are known. Insufficiency of RAN-binding protein-2 (RANBP2), a large, mosaic protein with pleiotropic functions, suppresses apoptosis of photoreceptor neurons upon aging and light-elicited oxidative stress, and promotes age-dependent tumorigenesis by mechanisms that are not well understood. Here we show that, by downregulating selective partners of RANBP2, such as RAN GTPase, UBC9 and ErbB-2 (HER2; Neu), and blunting the upregulation of a set of orphan nuclear receptors and the light-dependent accumulation of ubiquitylated substrates, light-elicited oxidative stress and Ranbp2 haploinsufficiency have a selective effect on protein homeostasis in the retina. Among the nuclear orphan receptors affected by insufficiency of RANBP2, we identified an isoform of COUP-TFI (Nr2f1) as the only receptor stably co-associating in vivo with RANBP2 and distinct isoforms of UBC9. Strikingly, most changes in proteostasis caused by insufficiency of RANBP2 in the retina are not observed in the supporting tissue, the retinal pigment epithelium (RPE). Instead, insufficiency of RANBP2 in the RPE prominently suppresses the light-dependent accumulation of lipophilic deposits, and it has divergent effects on the accumulation of free cholesterol and free fatty acids despite the genotype-independent increase of light-elicited oxidative stress in this tissue. Thus, the data indicate that insufficiency of RANBP2 results in the cell-type-dependent downregulation of protein and lipid homeostasis, acting on functionally interconnected pathways in response to oxidative stress. These results provide a rationale for the neuroprotection from light damage of photosensory neurons by RANBP2 insufficiency and for the identification of novel therapeutic targets and approaches promoting neuroprotection.
Figures

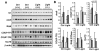

Similar articles
-
Selective impairment of a subset of Ran-GTP-binding domains of ran-binding protein 2 (Ranbp2) suffices to recapitulate the degeneration of the retinal pigment epithelium (RPE) triggered by Ranbp2 ablation.J Biol Chem. 2014 Oct 24;289(43):29767-89. doi: 10.1074/jbc.M114.586834. Epub 2014 Sep 3. J Biol Chem. 2014. PMID: 25187515 Free PMC article.
-
Impairments in age-dependent ubiquitin proteostasis and structural integrity of selective neurons by uncoupling Ran GTPase from the Ran-binding domain 3 of Ranbp2 and identification of novel mitochondrial isoforms of ubiquitin-conjugating enzyme E2I (ubc9) and Ranbp2.Small GTPases. 2019 Mar;10(2):146-161. doi: 10.1080/21541248.2017.1356432. Epub 2017 Sep 29. Small GTPases. 2019. PMID: 28877029 Free PMC article.
-
Uncoupling phototoxicity-elicited neural dysmorphology and death by insidious function and selective impairment of Ran-binding protein 2 (Ranbp2).FEBS Lett. 2015 Dec 21;589(24 Pt B):3959-68. doi: 10.1016/j.febslet.2015.11.037. Epub 2015 Nov 26. FEBS Lett. 2015. PMID: 26632511 Free PMC article.
-
Retinal Pigment Epithelium Under Oxidative Stress: Chaperoning Autophagy and Beyond.Int J Mol Sci. 2025 Jan 30;26(3):1193. doi: 10.3390/ijms26031193. Int J Mol Sci. 2025. PMID: 39940964 Free PMC article. Review.
-
RANBP2 evolution and human disease.FEBS Lett. 2023 Oct;597(20):2519-2533. doi: 10.1002/1873-3468.14749. Epub 2023 Oct 15. FEBS Lett. 2023. PMID: 37795679 Review.
Cited by
-
Dynamics and diverse functions of nuclear pore complex proteins.Nucleus. 2012 Mar 1;3(2):162-71. doi: 10.4161/nucl.19674. Epub 2012 Mar 1. Nucleus. 2012. PMID: 22555605 Free PMC article. Review.
-
Distinct and atypical intrinsic and extrinsic cell death pathways between photoreceptor cell types upon specific ablation of Ranbp2 in cone photoreceptors.PLoS Genet. 2013 Jun;9(6):e1003555. doi: 10.1371/journal.pgen.1003555. Epub 2013 Jun 20. PLoS Genet. 2013. PMID: 23818861 Free PMC article.
-
Proteomic Profiling of Neuroblastoma Cells Adhesion on Hyaluronic Acid-Based Surface for Neural Tissue Engineering.Biomed Res Int. 2016;2016:1917394. doi: 10.1155/2016/1917394. Epub 2016 Dec 7. Biomed Res Int. 2016. PMID: 28053978 Free PMC article.
-
Differential loss of prolyl isomerase or chaperone activity of Ran-binding protein 2 (Ranbp2) unveils distinct physiological roles of its cyclophilin domain in proteostasis.J Biol Chem. 2014 Feb 21;289(8):4600-25. doi: 10.1074/jbc.M113.538215. Epub 2014 Jan 8. J Biol Chem. 2014. PMID: 24403063 Free PMC article.
-
Selective impairment of a subset of Ran-GTP-binding domains of ran-binding protein 2 (Ranbp2) suffices to recapitulate the degeneration of the retinal pigment epithelium (RPE) triggered by Ranbp2 ablation.J Biol Chem. 2014 Oct 24;289(43):29767-89. doi: 10.1074/jbc.M114.586834. Epub 2014 Sep 3. J Biol Chem. 2014. PMID: 25187515 Free PMC article.
References
-
- Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL. (2006). SUMO1 haploinsufficiency leads to cleft lip and palate. Science 313, 1751. - PubMed
-
- Andersen JK. (2004). Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 10, S18–S25 - PubMed
-
- Bazan NG. (2006). Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 29, 263–271 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

