Post-translational maturation of dystroglycan is necessary for pikachurin binding and ribbon synaptic localization
- PMID: 20682766
- PMCID: PMC2951195
- DOI: 10.1074/jbc.M110.116343
Post-translational maturation of dystroglycan is necessary for pikachurin binding and ribbon synaptic localization
Abstract
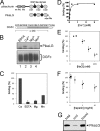
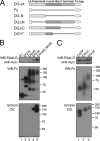
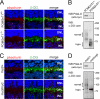
Pikachurin, the most recently identified ligand of dystroglycan, plays a crucial role in the formation of the photoreceptor ribbon synapse. It is known that glycosylation of dystroglycan is necessary for its ligand binding activity, and hypoglycosylation is associated with a group of muscular dystrophies that often involve eye abnormalities. Because little is known about the interaction between pikachurin and dystroglycan and its impact on molecular pathogenesis, here we characterize the interaction using deletion constructs and mouse models of muscular dystrophies with glycosylation defects (Large(myd) and POMGnT1-deficient mice). Pikachurin-dystroglycan binding is calcium-dependent and relatively less sensitive to inhibition by heparin and high NaCl concentration, as compared with other dystroglycan ligand proteins. Using deletion constructs of the laminin globular domains in the pikachurin C terminus, we show that a certain steric structure formed by the second and the third laminin globular domains is necessary for the pikachurin-dystroglycan interaction. Binding assays using dystroglycan deletion constructs and tissue samples from Large-deficient (Large(myd)) mice show that Large-dependent modification of dystroglycan is necessary for pikachurin binding. In addition, the ability of pikachurin to bind to dystroglycan prepared from POMGnT1-deficient mice is severely reduced, suggesting that modification of the GlcNAc-β1,2-branch on O-mannose is also necessary for the interaction. Immunofluorescence analysis reveals a disruption of pikachurin localization in the photoreceptor ribbon synapse of these model animals. Together, our data demonstrate that post-translational modification on O-mannose, which is mediated by Large and POMGnT1, is essential for pikachurin binding and proper localization, and suggest that their disruption underlies the molecular pathogenesis of eye abnormalities in a group of muscular dystrophies.
Figures


Similar articles
-
Pikachurin interaction with dystroglycan is diminished by defective O-mannosyl glycosylation in congenital muscular dystrophy models and rescued by LARGE overexpression.Neurosci Lett. 2011 Feb 1;489(1):10-5. doi: 10.1016/j.neulet.2010.11.056. Epub 2010 Dec 1. Neurosci Lett. 2011. PMID: 21129441 Free PMC article.
-
Presynaptic dystroglycan-pikachurin complex regulates the proper synaptic connection between retinal photoreceptor and bipolar cells.J Neurosci. 2012 May 2;32(18):6126-37. doi: 10.1523/JNEUROSCI.0322-12.2012. J Neurosci. 2012. PMID: 22553019 Free PMC article.
-
Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation.Nat Neurosci. 2008 Aug;11(8):923-31. doi: 10.1038/nn.2160. Epub 2008 Jul 20. Nat Neurosci. 2008. PMID: 18641643
-
[Essential role of pikachurin, a novel dystroglycan-binding protein, in bipolar dendrite connection to photoreceptor ribbon synapse in the retina].Nippon Ganka Gakkai Zasshi. 2010 Nov;114(11):955-67. Nippon Ganka Gakkai Zasshi. 2010. PMID: 21141075 Review. Japanese.
-
[Pathomechanism and therapeutic strategy of Fukuyama congenital muscular dystrophy and related disorders].Rinsho Shinkeigaku. 2009 Nov;49(11):859-62. doi: 10.5692/clinicalneurol.49.859. Rinsho Shinkeigaku. 2009. PMID: 20030231 Review. Japanese.
Cited by
-
Postnatal Gene Therapy Improves Spatial Learning Despite the Presence of Neuronal Ectopia in a Model of Neuronal Migration Disorder.Genes (Basel). 2016 Nov 29;7(12):105. doi: 10.3390/genes7120105. Genes (Basel). 2016. PMID: 27916859 Free PMC article.
-
From adhesion complex to signaling hub: the dual role of dystroglycan.Front Mol Biosci. 2023 Dec 14;10:1325284. doi: 10.3389/fmolb.2023.1325284. eCollection 2023. Front Mol Biosci. 2023. PMID: 38155958 Free PMC article. Review.
-
Dystroglycan-HSPG interactions provide synaptic plasticity and specificity.Glycobiology. 2024 Aug 30;34(10):cwae051. doi: 10.1093/glycob/cwae051. Glycobiology. 2024. PMID: 39223703 Free PMC article. Review.
-
Eyes shut homolog (EYS) interacts with matriglycan of O-mannosyl glycans whose deficiency results in EYS mislocalization and degeneration of photoreceptors.Sci Rep. 2020 May 8;10(1):7795. doi: 10.1038/s41598-020-64752-4. Sci Rep. 2020. PMID: 32385361 Free PMC article.
-
Cellular and Molecular Mechanisms Regulating Retinal Synapse Development.Annu Rev Vis Sci. 2024 Sep;10(1):377-402. doi: 10.1146/annurev-vision-102122-105721. Annu Rev Vis Sci. 2024. PMID: 39292551 Free PMC article. Review.
References
-
- Barresi R., Campbell K. P. (2006) J. Cell. Sci. 119, 199–207 - PubMed
-
- Cohn R. D., Henry M. D., Michele D. E., Barresi R., Saito F., Moore S. A., Flanagan J. D., Skwarchuk M. W., Robbins M. E., Mendell J. R., Williamson R. A., Campbell K. P. (2002) Cell 110, 639–648 - PubMed
-
- Moore S. A., Saito F., Chen J., Michele D. E., Henry M. D., Messing A., Cohn R. D., Ross-Barta S. E., Westra S., Williamson R. A., Hoshi T., Campbell K. P. (2002) Nature 418, 422–425 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

