Growth hormone (GH)-dependent expression of a natural antisense transcript induces zinc finger E-box-binding homeobox 2 (ZEB2) in the glomerular podocyte: a novel action of gh with implications for the pathogenesis of diabetic nephropathy
- PMID: 20682777
- PMCID: PMC2951188
- DOI: 10.1074/jbc.M110.132332
Growth hormone (GH)-dependent expression of a natural antisense transcript induces zinc finger E-box-binding homeobox 2 (ZEB2) in the glomerular podocyte: a novel action of gh with implications for the pathogenesis of diabetic nephropathy
Abstract
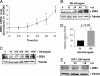
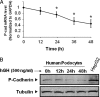
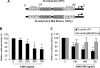
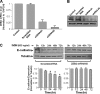
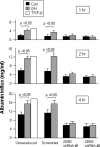
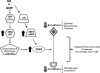
Growth hormone (GH) excess results in structural and functional changes in the kidney and is implicated as a causative factor in the development of diabetic nephropathy (DN). Glomerular podocytes are the major barrier to the filtration of serum proteins, and altered podocyte function and/or reduced podocyte number is a key event in the pathogenesis of DN. We have previously shown that podocytes are a target for GH action. To elucidate the molecular basis for the effects of GH on the podocyte, we conducted microarray and RT-quantitative PCR analyses of immortalized human podocytes and identified zinc finger E-box-binding homeobox 2 (ZEB2) to be up-regulated in a GH dose- and time-dependent manner. We established that the GH-dependent increase in ZEB2 levels is associated with increased transcription of a ZEB2 natural antisense transcript required for efficient translation of the ZEB2 transcript. GH down-regulated expression of E- and P-cadherins, targets of ZEB2, and inhibited E-cadherin promoter activity. Mutation of ZEB2 binding sites on the E-cadherin promoter abolished this effect of GH on the E-cadherin promoter. Whereas GH increased podocyte permeability to albumin in a paracellular albumin influx assay, shRNA-mediated knockdown of ZEB2 expression abrogated this effect. We conclude that GH increases expression of ZEB2 in part by increasing expression of a ZEB2 natural antisense transcript. GH-dependent increase in ZEB2 expression results in loss of P- and E-cadherins in podocytes and increased podocyte permeability to albumin. Decreased expression of P- and E-cadherins is implicated in podocyte dysfunction and epithelial-mesenchymal transition observed in DN. We speculate that the actions of GH on ZEB2 and P- and E-cadherin expression play a role in the pathogenesis of microalbuminuria of DN.
Figures


Similar articles
-
Novel Actions of Growth Hormone in Podocytes: Implications for Diabetic Nephropathy.Front Med (Lausanne). 2017 Jul 12;4:102. doi: 10.3389/fmed.2017.00102. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28748185 Free PMC article. Review.
-
Growth Hormone Induces Transforming Growth Factor-Beta-Induced Protein in Podocytes: Implications for Podocyte Depletion and Proteinuria.J Cell Biochem. 2015 Sep;116(9):1947-56. doi: 10.1002/jcb.25150. J Cell Biochem. 2015. PMID: 25740786
-
Carboxymethyl lysine induces EMT in podocytes through transcription factor ZEB2: Implications for podocyte depletion and proteinuria in diabetes mellitus.Arch Biochem Biophys. 2016 Jan 15;590:10-19. doi: 10.1016/j.abb.2015.11.003. Epub 2015 Nov 10. Arch Biochem Biophys. 2016. PMID: 26550927
-
Hypoxia induces ZEB2 in podocytes: Implications in the pathogenesis of proteinuria.J Cell Physiol. 2019 May;234(5):6503-6518. doi: 10.1002/jcp.27387. Epub 2018 Sep 21. J Cell Physiol. 2019. PMID: 30238984
-
The glomerular podocyte as a target of growth hormone action: implications for the pathogenesis of diabetic nephropathy.Curr Diabetes Rev. 2011 Jan;7(1):50-5. doi: 10.2174/157339911794273900. Curr Diabetes Rev. 2011. PMID: 21067510 Free PMC article. Review.
Cited by
-
Cell biology and pathology of podocytes.Annu Rev Physiol. 2012;74:299-323. doi: 10.1146/annurev-physiol-020911-153238. Epub 2011 Nov 4. Annu Rev Physiol. 2012. PMID: 22054238 Free PMC article. Review.
-
Insight into the Molecular Mechanism of Diabetic Kidney Disease and the Role of Metformin in Its Pathogenesis.Int J Mol Sci. 2023 Aug 22;24(17):13038. doi: 10.3390/ijms241713038. Int J Mol Sci. 2023. PMID: 37685845 Free PMC article. Review.
-
Metabolic syndrome and associated chronic kidney diseases: nutritional interventions.Rev Endocr Metab Disord. 2013 Sep;14(3):273-86. doi: 10.1007/s11154-013-9268-2. Rev Endocr Metab Disord. 2013. PMID: 24036690 Review.
-
Novel Actions of Growth Hormone in Podocytes: Implications for Diabetic Nephropathy.Front Med (Lausanne). 2017 Jul 12;4:102. doi: 10.3389/fmed.2017.00102. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28748185 Free PMC article. Review.
-
Comparative Analysis of Promoters and Enhancers in the Pituitary Glands of the Bama Xiang and Large White Pigs.Front Genet. 2021 Jul 23;12:697994. doi: 10.3389/fgene.2021.697994. eCollection 2021. Front Genet. 2021. PMID: 34367256 Free PMC article.
References
-
- Møller N., Jørgensen J. O. (2009) Endocr. Rev. 30, 152–177 - PubMed
-
- Lu C., Schwartzbauer G., Sperling M. A., Devaskar S. U., Thamotharan S., Robbins P. D., McTiernan C. F., Liu J. L., Jiang J., Frank S. J., Menon R. K. (2001) J. Biol. Chem. 276, 22892–22900 - PubMed
-
- Baudet M. L., Rattray D., Martin B. T., Harvey S. (2009) Endocrinology 150, 2758–2766 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

