Association among amyloid plaque, lipid, and creatine in hippocampus of TgCRND8 mouse model for Alzheimer disease
- PMID: 20682779
- PMCID: PMC2951194
- DOI: 10.1074/jbc.M110.142174
Association among amyloid plaque, lipid, and creatine in hippocampus of TgCRND8 mouse model for Alzheimer disease
Abstract
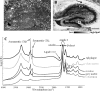
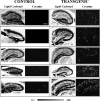
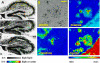
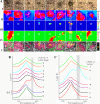
Amyloid peptide (Aβ) aggregation in the brain is a characteristic feature of Alzheimer disease (AD). Previously, we reported the discovery of focally elevated creatine deposits in brain tissue from TgCRND8 mice, which express double mutant (K670N/M671L and V717F) amyloid protein precursor. In this study, frozen hippocampal tissue sections from 5-, 8-, 11-, 14-, and 17-month old TgCRND8 and littermate control mice were examined with Fourier transform infrared microspectroscopy to explore the distribution of lipid, creatine, and dense core plaque deposits. Lipid distribution throughout the hippocampus was similar in transgenic (Tg) and non-Tg littermates at all ages. Dense core plaques were always found to lie within a thin (30-50 μm) lipid envelope, confirmed by imaging through serial sections. Creatine deposits were found in all TgCRND8 mice; the extent of deposition increased with age. Minor creatine deposits appeared in the oldest littermate controls. Distribution in the serial sections showed moderate correlation between layers, slightly disturbed by the freeze/thaw process. Creatine deposits in Tg mice were not specifically co-localized with plaques or lipid halos. The dimension of the lipid envelope is comparable with that of the diffuse halo of nonaggregated amyloid, implying a dynamic association in vivo, postulated to have a significant role in the evolving neurotoxicity.
Figures
References
-
- Braak H., Braak E. (1998) J. Neural Transm. 53, (suppl) 127–140 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

