Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring
- PMID: 20682841
- PMCID: PMC2980359
- DOI: 10.1152/ajpendo.00058.2010
Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring
Abstract
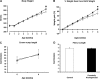
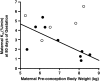
Discrete fetal androgen excess during early gestation in rhesus monkeys (Macaca mulatta) promotes endocrine antecedents of adult polycystic ovary syndrome (PCOS)-like traits in female offspring. Because developmental changes promoting such PCOS-like metabolic dysfunction remain unclear, the present study examined time-mated, gravid rhesus monkeys with female fetuses, of which nine gravid females received 15 mg of testosterone propionate (TP) subcutaneously daily from 40 to 80 days (first to second trimesters) of gestation [term, mean (range): 165 (155-175) days], whereas an additional six such females received oil vehicle injections over the same time interval. During gestation, ultrasonography quantified fetal growth measures and was used as an adjunct for fetal blood collections. At term, all fetuses were delivered by cesarean section for postnatal studies. Blood samples were collected from dams and infants for glucose, insulin, and total free fatty acid (FFA) determinations. TP injections transiently accelerated maternal weight gain in dams, very modestly increased head diameter of prenatally androgenized (PA) fetuses, and modestly increased weight gain in infancy compared with concurrent controls. Mild to moderate glucose intolerance, with increased area-under-the-curve circulating insulin values, occurred in TP-injected dams during an intravenous glucose tolerance test in the early second trimester. Moreover, reduced circulating FFA levels occurred in PA fetuses during a third trimester intravenous glucagon-tolbutamide challenge (140 days gestation), whereas excessive insulin sensitivity and increased insulin secretion relative to insulin sensitivity occurred in PA infants during an intravenous glucose-tolbutamide test at ∼1.5 mo postnatal age. Data from these studies suggest that experimentally induced fetal androgen excess may result in transient hyperglycemic episodes in the intrauterine environment that are sufficient to induce relative increases in pancreatic function in PA infants, suggesting in this nonhuman primate model that differential programming of insulin action and secretion may precede adult metabolic dysfunction.
Figures

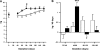
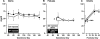
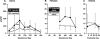
References
-
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 11: 357–374, 2005 - PubMed
-
- Abbott DH, Bruns CM, Barnett DK, Dumesic DA. Fetal programming of polycystic ovary syndrome ( 2nd ed.). In: Polycystic Ovary Syndrome, edited by Kovacs WG, Norman RL. Cambridge, UK: Cambridge Univ Press, 2007, p. 262–287
-
- Abbott DH, Dumesic DA. Fetal androgen excess provides a developmental origin for polycystic ovary syndrome. Expert Rev Obstet Gynecol 4: 1–7, 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

