Expression and distribution of voltage-gated ion channels in ferret sinoatrial node
- PMID: 20682846
- PMCID: PMC2957791
- DOI: 10.1152/physiolgenomics.00049.2010
Expression and distribution of voltage-gated ion channels in ferret sinoatrial node
Abstract
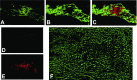
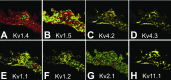
Spontaneous diastolic depolarization in the sinoatrial (SA) node enables it to serve as pacemaker of the heart. The variable cell morphology within the SA node predicts that ion channel expression would be heterogeneous and different from that in the atrium. To evaluate ion channel heterogeneity within the SA node, we used fluorescent in situ hybridization to examine ion channel expression in the ferret SA node region and atrial appendage. SA nodal cells were distinguished from surrounding cardiac myocytes by expression of the slow (SA node) and cardiac (surrounding tissue) forms of troponin I. Nerve cells in the sections were identified by detection of GAP-43 and cytoskeletal middle neurofilament. Transcript expression was characterized for the 4 hyperpolarization-activated cation channels, 6 voltage-gated Na(+) channels, 3 voltage-gated Ca(2+) channels, 24 voltage-gated K(+) channel α-subunits, and 3 ancillary subunits. To ensure that transcript expression was representative of protein expression, immunofluorescence was used to verify localization patterns of voltage-dependent K(+) channels. Colocalizations were performed to observe any preferential patterns. Some overlapping and nonoverlapping binding patterns were observed. Measurement of different cation channel transcripts showed heterogeneous expression with many different patterns of expression, attesting to the complexity of electrical activity in the SA node. This study provides insight into the possible role ion channel heterogeneity plays in SA node pacemaker activity.
Figures






Similar articles
-
Heterogeneity in K+ channel transcript expression detected in isolated ferret cardiac myocytes.Pacing Clin Electrophysiol. 1997 Feb;20(2 Pt 2):388-96. doi: 10.1111/j.1540-8159.1997.tb06198.x. Pacing Clin Electrophysiol. 1997. PMID: 9058843
-
Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node.J Biol Chem. 1999 Apr 30;274(18):12835-9. doi: 10.1074/jbc.274.18.12835. J Biol Chem. 1999. PMID: 10212270
-
Sino-atrial nodal cells of mammalian hearts: ionic currents and gene expression of pacemaker ionic channels.J Smooth Muscle Res. 2003 Oct;39(5):175-93. doi: 10.1540/jsmr.39.175. J Smooth Muscle Res. 2003. PMID: 14695028 Review.
-
Differential expression of ion channel transcripts in atrial muscle and sinoatrial node in rabbit.Circ Res. 2006 Dec 8;99(12):1384-93. doi: 10.1161/01.RES.0000251717.98379.69. Epub 2006 Nov 2. Circ Res. 2006. PMID: 17082478
-
Sustained inward current during pacemaker depolarization in mammalian sinoatrial node cells.Circ Res. 2000 Jul 21;87(2):88-91. doi: 10.1161/01.res.87.2.88. Circ Res. 2000. PMID: 10903990 Review.
Cited by
-
Augmentation of myocardial If dysregulates calcium homeostasis and causes adverse cardiac remodeling.Nat Commun. 2019 Jul 23;10(1):3295. doi: 10.1038/s41467-019-11261-2. Nat Commun. 2019. PMID: 31337768 Free PMC article.
-
Mitochondrial dysfunction is a key link involved in the pathogenesis of sick sinus syndrome: a review.Front Cardiovasc Med. 2024 Oct 29;11:1488207. doi: 10.3389/fcvm.2024.1488207. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39534498 Free PMC article. Review.
-
Conotoxin κM-RIIIJ, a tool targeting asymmetric heteromeric Kv1 channels.Proc Natl Acad Sci U S A. 2019 Jan 15;116(3):1059-1064. doi: 10.1073/pnas.1813161116. Epub 2018 Dec 28. Proc Natl Acad Sci U S A. 2019. PMID: 30593566 Free PMC article.
-
Comparative Study of Transcriptome in the Hearts Isolated from Mice, Rats, and Humans.Biomolecules. 2022 Jun 20;12(6):859. doi: 10.3390/biom12060859. Biomolecules. 2022. PMID: 35740984 Free PMC article.
-
Bidirectional flow of the funny current (If) during the pacemaking cycle in murine sinoatrial node myocytes.Proc Natl Acad Sci U S A. 2021 Jul 13;118(28):e2104668118. doi: 10.1073/pnas.2104668118. Proc Natl Acad Sci U S A. 2021. PMID: 34260402 Free PMC article.
References
-
- Augood SJ, Arbuthnott GW, Emson PC. Identified cholinergic neurones in the adult rat brain are enriched in GAP-43 mRNA: a double in situ hybridisation study. J Chem Neuroanat 9: 17–26, 1995. - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and IsK (minK) proteins associate for form the IKs cardiac potassium current. Nature 384: 78–80, 1996. - PubMed
-
- Benvenuti LA, Aiello VD, Higuchi MD. Different cell types within the sinoatrial node. Circulation 100: 1011–1012, 1999. - PubMed
-
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev 84: 803–833, 2004. - PubMed
-
- Bleeker WK, Mackaay AJC, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res 46: 11–22, 1980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

