Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: identification of infected snails from early prepatency
- PMID: 20682894
- PMCID: PMC2911197
- DOI: 10.4269/ajtmh.2010.09-0764
Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: identification of infected snails from early prepatency
Abstract
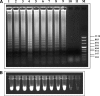
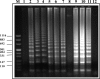
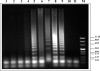
Monitoring post-control transmission of schistosomes by examining humans becomes less effective as infection rates among humans decrease. Molecular monitoring of prepatent schistosome infection in snails by the polymerase chain reaction (PCR) has been used for studying human-to-snail transmission, and snail prepatent infection rates were found to correspond to infection prevalence and average intensity in human populations contacting the sites studied. We have now developed loop-mediated isothermal amplification (LAMP) assays for identifying Schistosoma mansoni and S. haematobium to facilitate large-scale evaluation of post-intervention transmission potential. LAMP primers were designed based on the Sm1-7 and DraI repeated sequences of the corresponding schistosomes, and amplification by LAMP of these 121-basepair highly abundant sequences provided a detection sensitivity of 0.1 fg of genomic DNA. When these LAMP assays were applied for examining infected laboratory snails, it was possible to identify infection from the first day after exposure to miracidia. The potential advantages of these assays are discussed.
Figures


Similar articles
-
Biompha-LAMP: A New Rapid Loop-Mediated Isothermal Amplification Assay for Detecting Schistosoma mansoni in Biomphalaria glabrata Snail Host.PLoS Negl Trop Dis. 2016 Dec 12;10(12):e0005225. doi: 10.1371/journal.pntd.0005225. eCollection 2016 Dec. PLoS Negl Trop Dis. 2016. PMID: 27941967 Free PMC article.
-
Detection and quantification of schistosome DNA in freshwater snails using either fluorescent probes in real-time PCR or oligochromatographic dipstick assays targeting the ribosomal intergenic spacer.Acta Trop. 2013 Nov;128(2):241-9. doi: 10.1016/j.actatropica.2011.10.019. Epub 2011 Nov 11. Acta Trop. 2013. PMID: 22100540
-
Schistosoma species detection by environmental DNA assays in African freshwaters.PLoS Negl Trop Dis. 2020 Mar 23;14(3):e0008129. doi: 10.1371/journal.pntd.0008129. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32203507 Free PMC article.
-
Detecting and identifying Schistosoma infections in snails and aquatic habitats: A systematic review.PLoS Negl Trop Dis. 2021 Mar 24;15(3):e0009175. doi: 10.1371/journal.pntd.0009175. eCollection 2021 Mar. PLoS Negl Trop Dis. 2021. PMID: 33760814 Free PMC article.
-
A new surveillance and response tool: risk map of infected Oncomelania hupensis detected by Loop-mediated isothermal amplification (LAMP) from pooled samples.Acta Trop. 2015 Jan;141(Pt B):170-7. doi: 10.1016/j.actatropica.2014.01.006. Epub 2014 Feb 2. Acta Trop. 2015. PMID: 24495631 Review.
Cited by
-
Diagnosis of brugian filariasis by loop-mediated isothermal amplification.PLoS Negl Trop Dis. 2012;6(12):e1948. doi: 10.1371/journal.pntd.0001948. Epub 2012 Dec 13. PLoS Negl Trop Dis. 2012. PMID: 23272258 Free PMC article.
-
Comparison of sensitivity and specificity of three diagnostic tests to detect Schistosoma mansoni infections in school children in Mwanza region, Tanzania.PLoS One. 2018 Aug 22;13(8):e0202499. doi: 10.1371/journal.pone.0202499. eCollection 2018. PLoS One. 2018. PMID: 30133490 Free PMC article. Clinical Trial.
-
Snail-Related Contributions from the Schistosomiasis Consortium for Operational Research and Evaluation Program Including Xenomonitoring, Focal Mollusciciding, Biological Control, and Modeling.Am J Trop Med Hyg. 2020 Jul;103(1_Suppl):66-79. doi: 10.4269/ajtmh.19-0831. Am J Trop Med Hyg. 2020. PMID: 32400353 Free PMC article. Review.
-
Loop-Mediated Isothermal Amplification as Point-of-Care Diagnosis for Neglected Parasitic Infections.Int J Mol Sci. 2020 Oct 28;21(21):7981. doi: 10.3390/ijms21217981. Int J Mol Sci. 2020. PMID: 33126446 Free PMC article. Review.
-
Development of a rapid and highly sensitive nucleic acid-based diagnostic test for schistosomes, leveraging on identical multi-repeat sequences.Front Parasitol. 2024 Mar 14;3:1361493. doi: 10.3389/fpara.2024.1361493. eCollection 2024. Front Parasitol. 2024. PMID: 39817162 Free PMC article.
References
-
- Rabarijaona LP, Boisier P, Ravaoalimalala VE, Jeanne I, Roux JF, Jutand MA, Salamon R. Lot quality assurance sampling for screening communities hyperendemic for Schistosoma mansoni. Trop Med Int Health. 2003;8:322–328. - PubMed
-
- Lengeler C, Utzinger J, Tanner M. Screening of schistosomiasis with questionnaires. Trends Parasitol. 2002;18:375–377. - PubMed
-
- Fenwick A. New initiatives against Africa's worms. Trans R Soc Trop Med Hyg. 2006;100:200–207. - PubMed
-
- King C, Dickman K, Tisch DJ. Reassessment of the cost of chronic helminthic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

