Design of a bioartificial pancreas(+)
- PMID: 20683347
- PMCID: PMC2946508
- DOI: 10.231/JIM.0b013e3181ed3807
Design of a bioartificial pancreas(+)
Abstract
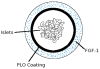
Introduction: In type 1 diabetes, the β-cells that secrete insulin have been destroyed such that daily exogenous insulin administration is required for the control of blood glucose in individuals with the disease. After the development of reliable techniques for the isolation of islets from the human pancreas, islet transplantation has emerged as a therapeutic option, albeit for only a few selected patients largely because there are not enough islets for the millions of patients requiring the treatment, and there is also the need to use immunosuppressive drugs to prevent transplant rejection. In 1980, the concept of islet immunoisolation by microencapsulation was introduced as a technique to overcome these 2 major barriers to islet transplantation. Microencapsulation of islets and transplantation in the peritoneal cavity was then described as a bioartificial pancreas. However, it is difficult to retrieve encapsulated islets transplanted in the peritoneal cavity, thus making it difficult to meet all the criteria for a bioartificial pancreas. A new design of a bioartificial pancreas comprising islets co-encapsulated with angiogenic protein in permselective multilayer alginate-poly-L-ornithine-alginate microcapsules and transplanted in an omentum pouch is described in this paper.
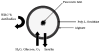
Materials and methods: The multilayer alginate-poly-L-ornithine-alginate microcapsules are made with ultrapure alginate using poly-L-ornithine as a semipermeable membrane separating the 2 alginate layers. The inner alginate layer is used to encapsulate the islets, and the outer layer is used to encapsulate angiogenic protein, which would induce neovascularization around the graft within the omentum pouch.
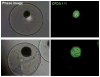
Results: In in vitro studies, we found that both the wild-type and the heparin-binding growth-associated molecule (HBGAM)-fibroblast growth factor-1 chimera can be encapsulated and released in a controlled and sustained manner from the outer alginate layer with a mean diameter in the range of 113 to 164 µm when 1.25% high guluronic acid alginate is used to formulate this outer layer.
Discussion: We are currently performing in vivo experiments to determine the ability of angiogenic proteins released from this outer layer to induce neovascularization around the grafts in the omentum pouch. We will subsequently examine the effect of co-encapsulation of islets with angiogenic protein on blood glucose control in diabetic animals. It is hoped that addition of tissue engineering to encapsulated islet transplantation will result in long-term survival of the islets and their ability to control blood glucose in type 1 diabetes without the necessity to use risky immunosuppressive drugs to prevent transplant rejection.
Figures

References
-
- Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. - PubMed
-
- Keller U. From obesity to diabetes. Int J Vitam Nutr Res. 2006;76:172–177. - PubMed
-
- Chukwuma C., Sr Type 1 diabetic nephropathy: Clinical characteristics and economic impact. J Diabetes and its Complications. 1993;7:15–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

