Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA
- PMID: 20683441
- PMCID: PMC2944060
- DOI: 10.1038/emboj.2010.179
Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA
Abstract
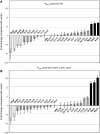
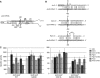
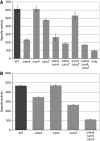
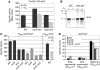
The alternative sigma factor RpoS responds to multiple stresses and activates a large number of genes that allow bacteria to adapt to changing environments. The accumulation of RpoS is regulated at multiple levels, including the regulation of its translation by small regulatory RNAs (sRNAs). A library of plasmids expressing each of 26 Escherichia coli sRNAs that bind Hfq was created to globally and rapidly analyse regulation of an rpoS-lacZ translational fusion. The approach can be easily applied to any gene of interest. When overexpressed, four sRNAs, including OxyS, previously shown to repress rpoS, were observed to repress the expression of the rpoS-lacZ fusion. Along with DsrA and RprA, two previously defined activators of rpoS translation, a third new sRNA activator, ArcZ, was identified. The expression of arcZ is repressed by the aerobic/anaerobic-sensing ArcA-ArcB two-component system under anaerobic conditions and adds translational regulation to the ArcA-ArcB regulon. ArcZ directly represses, and is repressed by, arcB transcription, providing a negative feedback loop that may affect functioning of the ArcA-ArcB regulon.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Aiso T, Ohki R (2003) Instability of sensory histidine kinase mRNAs in Escherichia coli. Genes Cells 8: 179–187 - PubMed
-
- Altuvia S, Weinstein-Fischer D, Zhang A, Postow L, Storz G (1997) A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90: 43–53 - PubMed
-
- Antal M, Bordeau V, Douchin V, Felden B (2005) A small bacterial RNA regulates a putative ABC transporter. J Biol Chem 280: 7901–7908 - PubMed
-
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S (2001) Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol 11: 941–950 - PubMed
-
- Bongaerts J, Zoske S, Weidner U, Unden G (1995) Transcriptional regulation of the proton translocating NADH dehydrogenase genes (nuoA–N) of Escherichia coli by electron acceptors, electron donors and gene regulators. Mol Microbiol 16: 521–534 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

