Bcr-abl silencing by specific small-interference RNA expression vector as a potential treatment for chronic myeloid leukemia
- PMID: 20683492
- PMCID: PMC3878140
Bcr-abl silencing by specific small-interference RNA expression vector as a potential treatment for chronic myeloid leukemia
Abstract
Background: RNA interference (RNAi) is the mechanism of gene silencing-mediated messenger RNA degradation by small interference RNA (siRNA), which becomes a powerful tool for in vivo research, especially in the areas of cancer. In this research, the potential use of an expression vector as a specific siRNA producing tool for silencing of Bcr-abl in K562 cell line has been investigated.
Methods: siRNA specific for Bcr-abl as short hairpin RNA (shRNA) was designed and cloned in expression vector (pRNAH1.1/Neo). K562 cells were cultured in RPMI media and transfected with shRNA expressing vector using lipofectamin 2000. Successful transfection was confirmed by significant increase of enhanced green fluorescent protein (EGFP) levels in K562-treated cells with expression vector (pEGFP-C1). In vitro studies in human K562 cell line entailed modulation of endogenous Bcr-abl mRNA levels which induced apoptosis. Effects of siRNA treatment on K562 cells were measured by ELISA.
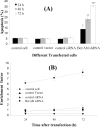
Results: Successful expression of siRNA was confirmed by significant reduction of Bcr-abl mRNA levels in K562 cells treated with expression vector (pRNAH1.1/Neo). siRNA directed against Bcr-abl effectively induced apoptosis and reduced viability in human K562 cell lines.
Conclusion: Expression vector of siRNA can be used in vitro to target specific RNA and to reduce the levels of the specific gene product in the targeted cells. Results of this work suggest that RNAi has potential application for the treatment of a variety of diseases, including those involving abnormal gene expression and viral contamination.
Figures




Similar articles
-
[Effect of specific siRNA targeting against bcr-abl chimeric gene on chronic myelogenous leukemia cells].Zhonghua Yi Xue Za Zhi. 2005 Jan 19;85(3):198-202. Zhonghua Yi Xue Za Zhi. 2005. PMID: 15854468 Chinese.
-
BCR/ABL mRNA targeting small interfering RNA effects on proliferation and apoptosis in chronic myeloid leukemia.Asian Pac J Cancer Prev. 2014;15(12):4773-80. doi: 10.7314/apjcp.2014.15.12.4773. Asian Pac J Cancer Prev. 2014. PMID: 24998540
-
Delivery of therapeutic shRNA and siRNA by Tat fusion peptide targeting BCR-ABL fusion gene in Chronic Myeloid Leukemia cells.J Control Release. 2010 Aug 3;145(3):272-80. doi: 10.1016/j.jconrel.2010.04.011. Epub 2010 Apr 24. J Control Release. 2010. PMID: 20403398
-
Short interfering RNA (siRNA) as a novel therapeutic.Clin Exp Pharmacol Physiol. 2006 May-Jun;33(5-6):504-10. doi: 10.1111/j.1440-1681.2006.04399.x. Clin Exp Pharmacol Physiol. 2006. Retraction in: Clin Exp Pharmacol Physiol. 2013 Apr;40(4):305. doi: 10.1111/1440-1681.12073. Retraction in: Clin Exp Pharmacol Physiol. 2013 Apr;40(4):305. doi: 10.1111/1440-1681.12075. PMID: 16700886 Retracted. Review.
-
Anti-c-myc RNAi-Based Onconanotherapeutics.Biomedicines. 2020 Dec 15;8(12):612. doi: 10.3390/biomedicines8120612. Biomedicines. 2020. PMID: 33333729 Free PMC article. Review.
Cited by
-
A case report of a 33-year chronic phase survivor of chronic myeloid leukemia.Med Oncol. 2012 Jun;29(2):1102-4. doi: 10.1007/s12032-011-9874-3. Epub 2011 Feb 27. Med Oncol. 2012. PMID: 21359860
-
ASP210: a potent oligonucleotide-based inhibitor effective against TKI-resistant CML cells.Am J Physiol Cell Physiol. 2024 Jul 1;327(1):C184-C192. doi: 10.1152/ajpcell.00188.2024. Epub 2024 Jun 3. Am J Physiol Cell Physiol. 2024. PMID: 38826137 Free PMC article.
-
A combination of STI571 and BCR-ABL1 siRNA with overexpressed p15INK4B induced enhanced proliferation inhibition and apoptosis in chronic myeloid leukemia.Braz J Med Biol Res. 2014 Dec;47(12):1096-101. doi: 10.1590/1414-431X20143734. Epub 2014 Oct 14. Braz J Med Biol Res. 2014. PMID: 25387678 Free PMC article.
References
-
- Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen M, Morten ØA, Hovgaard MB, Schmitz A, Nyengaard R, Besenbacher F, Kjems J. RNA interference in vitro and in vivo using a chitosan/siRNA nanoparticle system. Mol Ther. 2006;14(4):476–484. - PubMed
-
- Hannon GJ. RNA interference. Nature . 2002;418(6894):244–251. - PubMed
-
- Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9(3):347–351. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
