Cytotoxicity of subtoxic AgNP in human hepatoma cell line (HepG2) after long-term exposure
- PMID: 20683495
- PMCID: PMC3878143
Cytotoxicity of subtoxic AgNP in human hepatoma cell line (HepG2) after long-term exposure
Abstract
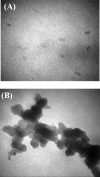
We aimed at evaluating the toxicity effects of low (subtoxic) concentrations of silver nanoparticles nanoparticles (AgNP, 5-10 nm) in human hepatoblastoma (HepG2) cell line after and during a period of about one month.
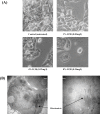
Methods: XTT and MTT assays were used to draw a dose-response curve; IC50 (half maximal inhibitory concentration) value of the AgNP on HepG2 cells was calculated to be 2.75-3.0 mg/l. The cells were exposed to concentrations of 0% (control), 1%, 4% and 8% IC50 of AgNP (corresponding to 0.00, 0.03, 0.12 and 0.24 mg/l of AgNP, respectively) for four consecutive passages. The treated cells were compared to the control group with respect to morphology and proliferation at the end of the period.
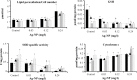
Results: The biochemical studies revealed significant increases of lactate dehydrogenase and alanine aminotransferase enzyme activity in the culture media of cells receiving 4% and 8% IC50; the increases in the aspartate aminotransferase enzyme activity and nitric oxide concentration became significant at 8% IC50. In the cell extracts, the average total protein and activity of glutathione peroxidase enzyme remained unchanged; the decrease in the average content of glutathione (GSH) and superoxide dismutase (SOD) activity became significant at 4% and 8% IC50. There were increases in lipid peroxidation (significant at 4% and 8% IC50) and cytochrome c content (significant at 8% IC50). The accumulations of the effects, during the experiment from one generation to the next, were not statistically remarkable except in cases of GSH and SOD. The results indicate clearly the involvement of oxidative changes in the cells after exposure to low doses of AgNP.
Conclusion: The results might help specify a safer amount of AgNP for use in different applications.
Figures



References
-
- Kreuter J. Nanoparticles-a historical perspective. Int J Pharm. 2007;331(1):1–10. - PubMed
-
- Maynard A D, Aitken R J, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert M A, Ryan J, Seaton A, Stone V, Tinkle S S, Tran L, Walker N J, Warheit D B. Safe handling of nanotechnology. Nature. 2006;444(7117):267–269. - PubMed
-
- Chen X, Schluesener H J. Nanosilver: a nanoproduct in medical application. Toxicol Lett . 2008;176(1):1–12. - PubMed
-
- Panyala N R, Pena-Mendez E N, Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health. J Appl Med. 2008;6:117–129.
-
- Silver S, Phung le T, Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J Ind Microbiol Biotechnol. 2006;33(7):627–634. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
