Administration of embryonic stem cells generates effective antitumor immunity in mice with minor and heavy tumor load
- PMID: 20683592
- PMCID: PMC11030618
- DOI: 10.1007/s00262-010-0899-9
Administration of embryonic stem cells generates effective antitumor immunity in mice with minor and heavy tumor load
Abstract
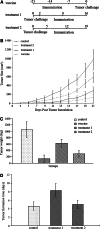
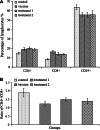
The history of immunizing animals with fetal tissues to generate an antitumor response dates back a century ago. Subsequent reports supported the idea that vaccination with embryonic materials could generate cancer-specific immunity and protect animals from transplantable and chemically induced tumors. In our study, we found C57 BL/6 mice vaccinated with embryonic stem cells (ESCs) received obvious antitumor immunity, which protected them from the formation and development of lung cancer. Furthermore, we investigated the antitumor effects of administration of ESCs in mice with minor and/or heavy tumor load. The tumor growth was monitored, the proliferation of lymphocytes and secretion of cytokines were examined, and finally the tissue sections were approached by immunohistochemical and apoptosis staining. The results suggested that mice injected with ESCs received obvious tumor inhibition and retardation due to significant lymphocyte proliferation and cytokine secretion, which help to rebuild the host's immunity against cancer to some extent and comprise the main part of antitumor immunity. Moreover, mice with minor tumor load received stronger antitumor effect compared with mice with heavy tumor load, may be due to relatively intact immune system. Thus, besides their function as prophylactic vaccines, administration of ESCs could be a potential treatment for cancer, which obviously prevent and control the proliferation and development of malignant tumors.
Conflict of interest statement
None.
Figures



Similar articles
-
Antitumor effect of embryonic stem cells in a non-small cell lung cancer model: antitumor factors and immune responses.Int J Med Sci. 2013 Aug 9;10(10):1314-20. doi: 10.7150/ijms.6538. eCollection 2013. Int J Med Sci. 2013. PMID: 23983591 Free PMC article.
-
Vaccination with embryonic stem cells generates effective antitumor immunity against ovarian cancer.Int J Mol Med. 2013 Jan;31(1):147-53. doi: 10.3892/ijmm.2012.1195. Epub 2012 Nov 22. Int J Mol Med. 2013. PMID: 23174760
-
Vaccination efficacy with marrow mesenchymal stem cell against cancer was enhanced under simulated microgravity.Biochem Biophys Res Commun. 2017 Apr 8;485(3):606-613. doi: 10.1016/j.bbrc.2017.01.136. Epub 2017 Feb 24. Biochem Biophys Res Commun. 2017. PMID: 28238782
-
Melittin-MIL-2 fusion protein as a candidate for cancer immunotherapy.J Transl Med. 2016 Jun 1;14(1):155. doi: 10.1186/s12967-016-0910-0. J Transl Med. 2016. PMID: 27246873 Free PMC article.
-
In situ vaccination: Harvesting low hanging fruit on the cancer immunotherapy tree.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019 Jan;11(1):e1524. doi: 10.1002/wnan.1524. Epub 2018 Apr 18. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019. PMID: 29667346 Review.
Cited by
-
Immunotargeting of cancer stem cells.Contemp Oncol (Pozn). 2015;19(1A):A52-9. doi: 10.5114/wo.2014.47129. Contemp Oncol (Pozn). 2015. PMID: 25691822 Free PMC article. Review.
-
Cancer vaccines: Looking to the future.Oncoimmunology. 2013 Mar 1;2(3):e23403. doi: 10.4161/onci.23403. Oncoimmunology. 2013. PMID: 23802081 Free PMC article.
-
Induced Pluripotent Stem Cell-Based Cancer Vaccines.Front Immunol. 2019 Jul 8;10:1510. doi: 10.3389/fimmu.2019.01510. eCollection 2019. Front Immunol. 2019. PMID: 31338094 Free PMC article. Review.
-
Immunobiology and signaling pathways of cancer stem cells: implication for cancer therapy.Cytotechnology. 2015 Oct;67(5):749-59. doi: 10.1007/s10616-014-9830-0. Epub 2014 Dec 17. Cytotechnology. 2015. PMID: 25516358 Free PMC article.
-
Placenta-derived gp96 as a multivalent prophylactic cancer vaccine.Sci Rep. 2013;3:1947. doi: 10.1038/srep01947. Sci Rep. 2013. PMID: 23739295 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

