Sympathetic neurons express and secrete MMP-2 and MT1-MMP to control nerve sprouting via pro-NGF conversion
- PMID: 20683769
- PMCID: PMC11498450
- DOI: 10.1007/s10571-010-9548-2
Sympathetic neurons express and secrete MMP-2 and MT1-MMP to control nerve sprouting via pro-NGF conversion
Abstract
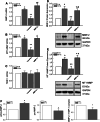
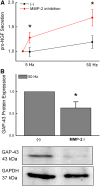
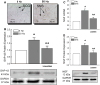
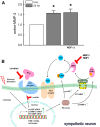
Recently, we have shown that high frequency electrical field stimulation (HFES) of sympathetic neurons (SN) induces nerve sprouting by up-regulation of nerve growth factor (NGF) which targets the tyrosine kinase A receptor (TrkA) in an autocrine/paracrine manner. There is increasing evidence that matrix metalloproteinase-2 (MMP-2) is not only involved in extracellular matrix (ECM) turnover but may also exert beneficial effects during neuronal growth. Therefore, this study aimed to investigate the regulation and function of MMP-2 and its major activator membrane type 1-matrix metalloproteinase (MT1-MMP) as well its inhibitor TIMP-1 in SN under conditions of HFES. Moreover, we analyzed molecular mechanisms of the beneficial effect of losartan, an angiotensin II type I receptor (AT-1)blocker on HFES-induced nerve sprouting. Cell cultures of SN from the superior cervical ganglia (SCG) of neonatal rats were electrically stimulated for 48 h with a frequency of 5 or 50 Hz. HFES increased MMP-2 and MT1-MMP mRNA and protein expression, whereas TIMP-1 expression remained unchanged. Under conditions of HFES, we observed a shift from pro- to active-MMP-2 indicating an increase in MMP-2 enzyme activity. Specific pharmacological MMP-2 inhibition contributed to an increase in pro-NGF amount in the cell culture supernatant and significantly reduced HFES-induced neurite outgrowth. Losartan abolished HFES-induced nerve sprouting in a significant manner by preventing HFES-induced NGF, MMP-2, and MT1-MMP up-regulation. In summary, specific MMP-2 blockade prevents sympathetic nerve sprouting (SNS) by inhibition of pro-NGF conversion while losartan abolishes HFES-induced SNS by reducing total NGF, MMP-2 and MT1-MMP expression.
Figures
Similar articles
-
Electrical stimulation of sympathetic neurons induces autocrine/paracrine effects of NGF mediated by TrkA.J Mol Cell Cardiol. 2010 Jul;49(1):79-87. doi: 10.1016/j.yjmcc.2010.01.019. Epub 2010 Feb 2. J Mol Cell Cardiol. 2010. PMID: 20138055
-
Mechanical stretch induces nerve sprouting in rat sympathetic neurocytes.Auton Neurosci. 2010 Jun 24;155(1-2):25-32. doi: 10.1016/j.autneu.2010.01.003. Epub 2010 Feb 1. Auton Neurosci. 2010. PMID: 20122881
-
Regulation of nerve growth factor in the heart: the role of the calcineurin-NFAT pathway.J Mol Cell Cardiol. 2009 Apr;46(4):568-78. doi: 10.1016/j.yjmcc.2008.12.006. Epub 2008 Dec 25. J Mol Cell Cardiol. 2009. PMID: 19150448
-
Rate and irregularity of electrical activation during atrial fibrillation affect myocardial NGF expression via different signalling routes.Cell Signal. 2012 Jan;24(1):99-105. doi: 10.1016/j.cellsig.2011.08.007. Epub 2011 Aug 26. Cell Signal. 2012. PMID: 21889978
-
Regulation of MT1-MMP and MMP-2 by the serpin PEDF: a promising new target for metastatic cancer.Cell Physiol Biochem. 2013;31(4-5):487-94. doi: 10.1159/000350069. Epub 2012 Mar 22. Cell Physiol Biochem. 2013. PMID: 23548673 Review.
Cited by
-
Neural functions of matrix metalloproteinases: plasticity, neurogenesis, and disease.Biochem Res Int. 2012;2012:789083. doi: 10.1155/2012/789083. Epub 2012 Apr 10. Biochem Res Int. 2012. PMID: 22567285 Free PMC article.
-
Toward an integrative analysis of the tumor microenvironment in ovarian epithelial carcinoma.Cancer Microenviron. 2012 Aug;5(2):173-83. doi: 10.1007/s12307-011-0092-5. Epub 2011 Nov 23. Cancer Microenviron. 2012. PMID: 22109660 Free PMC article.
-
A HPV16-related prognostic indicator for head and neck squamous cell carcinoma.Ann Transl Med. 2020 Nov;8(22):1492. doi: 10.21037/atm-20-6338. Ann Transl Med. 2020. PMID: 33313237 Free PMC article.
-
Sensory nerve-secreted factors regulate basal keratinocyte function in vitro.Integr Org Biol. 2025 Mar 3;7(1):obaf009. doi: 10.1093/iob/obaf009. eCollection 2025. Integr Org Biol. 2025. PMID: 40151298 Free PMC article.
-
Lectican proteoglycans, their cleaving metalloproteinases, and plasticity in the central nervous system extracellular microenvironment.Neuroscience. 2012 Aug 16;217:6-18. doi: 10.1016/j.neuroscience.2012.05.034. Epub 2012 May 22. Neuroscience. 2012. PMID: 22626649 Free PMC article. Review.
References
-
- Ahmad Z, Milligan CJ, Paton JF, Deuchars J (2003) Angiotensin type 1 receptor immunoreactivity in the thoracic spinal cord. Brain Res 985:21–31 - PubMed
-
- Borg TK, Caulfield JB (1981) The collagen matrix of the heart. Fed Proc 40:2037–2041 - PubMed
-
- Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS (2000) Nerve sprouting and sudden cardiac death. Circ Res 86:816–821 - PubMed
-
- Chang CM, Wu TJ, Zhou S, Doshi RN, Lee MH, Ohara T, Fishbein MC, Karagueuzian HS, Chen PS, Chen LS (2001) Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation 103:22–25 - PubMed
-
- Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY (2001) MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res 263:209–223 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

