Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis
- PMID: 20683959
- PMCID: PMC2917256
- DOI: 10.1002/hep.23679
Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis
Abstract
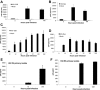
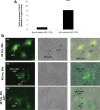
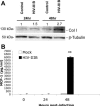
Patients coinfected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) develop more rapid fibrosis than those infected with HCV only. In HIV/HCV-coinfected patients, fibrosis progression correlates with HIV RNA levels, suggesting a direct role of HIV in liver fibrogenesis. Chemokine (C-C motif) receptor 5 (CCR5) and cysteine-X-cysteine receptor 4 (CXCR4), the two major coreceptors required for HIV entry into cells, are expressed on activated hepatic stellate cells (HSCs), the principle fibrogenic cell type in the liver. We therefore examined whether HIV can infect HSCs, explored the potential mechanisms of viral entry, and assessed the impact of infection as reflected by the ability of HSCs to transfer virus to T lymphocytes and elicit a proinflammatory and profibrogenic response. We report that the laboratory-adapted viruses HIV-IIIB (CXCR4-tropic or X4) and HIV-BaL (CCR5-tropic or R5) and primary HIV isolates can infect both a human stellate cell line, LX-2, and primary human HSCs. HIV entry and gene expression in HSCs was confirmed using HIV-green fluorescent protein (GFP) expression viral constructs in the presence or absence of the reverse-transcriptase inhibitor azidothymidine. CD4 expression on a subset of primary HSCs was demonstrated using fluorescence-activated cell sorting and immunofluorescence staining. Blocking experiments in the presence of anti-CD4, anti-CXCR4, and anti-CCR5 revealed that HIV entry into HSCs is predominantly CD4/chemokine coreceptor-independent. HIV infection promoted HSC collagen I expression and secretion of the proinflammatory cytokine monocyte chemoattractant protein-1. Furthermore, infected LX-2 cells were capable of transferring GFP-expressing virus to T lymphocytes in a coculture system.
Conclusion: Taken together, our results suggest a potential role of HIV in liver fibrosis/inflammation mediated through effects on HSCs. The role of early highly active antiretroviral therapy initiation in patients with HIV/HCV coinfection warrants further investigation.
Figures


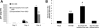
References
-
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. - PubMed
-
- Thomas D, Astemborski J, Rai R, Anania F, Schaeffer M, Galai N, et al. The natural history of hepatitic C infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. - PubMed
-
- Bonacini M, Govindarajan S, Blatt L, Schmid P, Conrad A, Lindsay K. Patients co-infected with human immunodeficiency virus and hepatitis C virus demonstrate higher levels of hepatic HCV RNA. J Viral Hepatitis. 1999;6:203–208. - PubMed
-
- Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. HEPATOLOGY. 1999;30:1054–1058. - PubMed
-
- Macias J, Berenguer J, Japon M, Giron J, Rivero A, Lopez-Cortes L, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. HEPATOLOGY. 2009;50:1056–1063. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
