Regulation of facial morphogenesis by endothelin signaling: insights from mice and fish
- PMID: 20684004
- PMCID: PMC2974943
- DOI: 10.1002/ajmg.a.33568
Regulation of facial morphogenesis by endothelin signaling: insights from mice and fish
Abstract
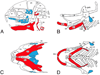
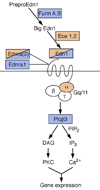
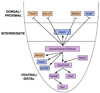
Craniofacial morphogenesis is accomplished through a complex set of developmental events, most of which are initiated in neural crest cells within the pharyngeal arches. Local patterning cues from the surrounding environment induce gene expression within neural crest cells, leading to formation of a diverse set of skeletal elements. Endothelin-1 (Edn1) is one of the primary signals that establishes the identity of neural crest cells within the mandibular portion of the first pharyngeal arch. Signaling through its cognate receptor, the endothelin-A receptor, is critical for patterning the ventral/distal portion of the arch (lower jaw) and also participates with Hox genes in patterning more posterior arches. Edn1/Ednra signaling is highly conserved between mouse and zebrafish, and genetic analyses in these two species have provided complementary insights into the patterning cues responsible for establishing the craniofacial complex as well as the genetic basis of facial birth defect syndromes.
Copyright © 2010 Wiley-Liss, Inc.
Figures
Similar articles
-
Requirements for Endothelin type-A receptors and Endothelin-1 signaling in the facial ectoderm for the patterning of skeletogenic neural crest cells in zebrafish.Development. 2007 Jan;134(2):335-45. doi: 10.1242/dev.02704. Epub 2006 Dec 13. Development. 2007. PMID: 17166927
-
Endothelin regulates neural crest deployment and fate to form great vessels through Dlx5/Dlx6-independent mechanisms.Mech Dev. 2013 Nov-Dec;130(11-12):553-66. doi: 10.1016/j.mod.2013.07.005. Epub 2013 Aug 8. Mech Dev. 2013. PMID: 23933587
-
Ectodermal-derived Endothelin1 is required for patterning the distal and intermediate domains of the mouse mandibular arch.Dev Biol. 2012 Nov 1;371(1):47-56. doi: 10.1016/j.ydbio.2012.08.003. Epub 2012 Aug 11. Dev Biol. 2012. PMID: 22902530 Free PMC article.
-
Understanding the basis of auriculocondylar syndrome: Insights from human, mouse and zebrafish genetic studies.Am J Med Genet C Semin Med Genet. 2013 Nov;163C(4):306-17. doi: 10.1002/ajmg.c.31376. Epub 2013 Oct 4. Am J Med Genet C Semin Med Genet. 2013. PMID: 24123988 Free PMC article. Review.
-
Understanding endothelin-1 function during craniofacial development in the mouse and zebrafish.Birth Defects Res C Embryo Today. 2004 Jun;72(2):190-9. doi: 10.1002/bdrc.20007. Birth Defects Res C Embryo Today. 2004. PMID: 15269892 Review.
Cited by
-
Evolution of the endothelin pathway drove neural crest cell diversification.Nature. 2020 Sep;585(7826):563-568. doi: 10.1038/s41586-020-2720-z. Epub 2020 Sep 16. Nature. 2020. PMID: 32939088
-
Embryonic expression of endothelins and their receptors in lamprey and frog reveals stem vertebrate origins of complex Endothelin signaling.Sci Rep. 2016 Sep 28;6:34282. doi: 10.1038/srep34282. Sci Rep. 2016. PMID: 27677704 Free PMC article.
-
Loss-of-function of Endothelin receptor type A results in Oro-Oto-Cardiac syndrome.Am J Med Genet A. 2020 May;182(5):1104-1116. doi: 10.1002/ajmg.a.61531. Epub 2020 Mar 5. Am J Med Genet A. 2020. PMID: 32133772 Free PMC article.
-
Hand1 phosphoregulation within the distal arch neural crest is essential for craniofacial morphogenesis.Development. 2014 Aug;141(15):3050-61. doi: 10.1242/dev.107680. Development. 2014. PMID: 25053435 Free PMC article.
-
Transcriptomic analysis reveals the role of SIX1 in mouse cranial neural crest patterning and bone development.Dev Dyn. 2023 Oct;252(10):1303-1315. doi: 10.1002/dvdy.597. Epub 2023 May 15. Dev Dyn. 2023. PMID: 37183792 Free PMC article.
References
-
- Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12:377–389. - PubMed
-
- Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–916. - PubMed
-
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

