WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity
- PMID: 20684029
- PMCID: PMC3179320
- DOI: 10.1002/jbmr.205
WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity
Abstract
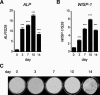
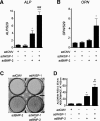
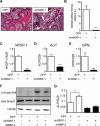
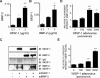
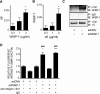
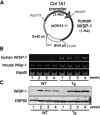
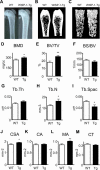
Wnt-induced secreted protein 1 (WISP-1/CCN4) is a member of the CCN family that is highly expressed in skeletal tissue and in osteoprogenitor cells induced to differentiate in vitro. To determine the function of WISP-1 during osteogeneis, osteogenic bone marrow stromal cells (BMSCs) were transduced with WISP-1 adenovirus (adWISP-1) in the presence or absence of bone morphogenetic protein 2 (BMP-2) adenovirus (adBMP-2). WISP-1 overexpression enhanced the ability of BMP-2 to direct BMSCs toward osteogenic differentiation and appeared to work by stimulating Smad-1/5/8 phosphorylation and activation. The ability of WISP-1 to enhance BMP-2 activity also was shown in vivo using an ectopic osteogenesis assay with BMSCs transduced with WISP-1, BMP-2, or both. When BMSCs were infected with lentivirus containing human WISP1 shRNA, they formed less bone in vivo and were less responsive to BMP-2, confirming that WISP-1 and BMP-2 have a functional interaction. Immunoprecipitation (IP) and Western blot analysis showed that WISP-1 bound directly to BMP-2 and showed that WISP-1 increased BMP-2 binding to hBMSCs in a dose-dependent fashion. To understand how WISP-1 enhanced BMP-2 signaling, the influence of WISP-1 on integrin expression was analyzed. WISP-1 induced the mRNA and protein levels of α(5)-integrin and, further, was found to bind to it. Antibody-blocking experiments showed that the BMP-2 binding to BMSCs that was enhanced by WISP-1 was completely neutralized by treatment with anti-integrin α(5)β(1) antibody. Pilot studies and the use of transgenic mice that overexpressed human WISP-1 in preosteoblasts had increased bone mineral density (BMD), trabecular thickness, and bone volume (BV/TV) over wild-type controls, supporting observations using human osteoprogenitors that WISP-1 has a positive influence on osteogenesis in vivo. In conclusion, these studies show, for the first time, that WISP-1 has a positive influence on bone cell differentiation and function and may work by enhancing the effects of BMP-2 to increase osteogenesis through a mechanism potentially involving binding to integrin α(5)β(1).
© 2011 American Society for Bone and Mineral Research.
Figures
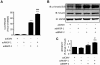


Similar articles
-
TGF-beta1 and WISP-1/CCN-4 can regulate each other's activity to cooperatively control osteoblast function.J Cell Biochem. 2008 Aug 1;104(5):1865-78. doi: 10.1002/jcb.21754. J Cell Biochem. 2008. PMID: 18404666 Free PMC article.
-
WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair.Am J Pathol. 2004 Sep;165(3):855-67. doi: 10.1016/S0002-9440(10)63348-2. Am J Pathol. 2004. PMID: 15331410 Free PMC article.
-
CCN4/WISP-1 positively regulates chondrogenesis by controlling TGF-β3 function.Bone. 2016 Feb;83:162-170. doi: 10.1016/j.bone.2015.11.007. Epub 2015 Nov 10. Bone. 2016. PMID: 26555637 Free PMC article.
-
The CCN family: a new stimulus package.J Endocrinol. 2003 Aug;178(2):169-75. doi: 10.1677/joe.0.1780169. J Endocrinol. 2003. PMID: 12904165 Review.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
Cited by
-
Perspectives on the role of nanotechnology in bone tissue engineering.Dent Mater. 2013 Jan;29(1):103-15. doi: 10.1016/j.dental.2012.08.001. Epub 2012 Aug 14. Dent Mater. 2013. PMID: 22901861 Free PMC article. Review.
-
Regulatory Effects and Interactions of the Wnt and OPG-RANKL-RANK Signaling at the Bone-Cartilage Interface in Osteoarthritis.Int J Mol Sci. 2019 Sep 19;20(18):4653. doi: 10.3390/ijms20184653. Int J Mol Sci. 2019. PMID: 31546898 Free PMC article. Review.
-
WISP-1 positively regulates angiogenesis by controlling VEGF-A expression in human osteosarcoma.Cell Death Dis. 2017 Apr 13;8(4):e2750. doi: 10.1038/cddis.2016.421. Cell Death Dis. 2017. PMID: 28406476 Free PMC article.
-
Analysis of CCN4 Function in Osteogenic and Osteoclastic Cells Using Gain and Loss of Function Approaches.Methods Mol Biol. 2017;1489:347-359. doi: 10.1007/978-1-4939-6430-7_29. Methods Mol Biol. 2017. PMID: 27734388 Free PMC article.
-
The Molecular Regulatory Mechanism in Multipotency and Differentiation of Wharton's Jelly Stem Cells.Int J Mol Sci. 2023 Aug 18;24(16):12909. doi: 10.3390/ijms241612909. Int J Mol Sci. 2023. PMID: 37629090 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

