Ca(2+) sensor GCAP1: A constitutive element of the ONE-GC-modulated odorant signal transduction pathway
- PMID: 20684533
- PMCID: PMC2936275
- DOI: 10.1021/bi101001v
Ca(2+) sensor GCAP1: A constitutive element of the ONE-GC-modulated odorant signal transduction pathway
Abstract
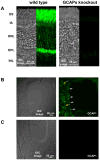
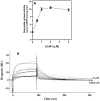
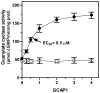
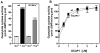
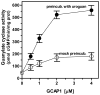
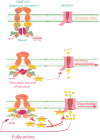
In a small subset of the olfactory sensory neurons, the odorant receptor ONE-GC guanylate cyclase is a central transduction component of the cyclic GMP signaling pathway. In a two-step transduction model, the odorant, uroguanylin, binds to the extracellular domain and activates its intracellular domain to generate the odorant second messenger, cyclic GMP. This study via comprehensive technology, including gene deletion, live cell Forster resonance energy transfer (FRET), and surface plasmon resonance (SPR) spectroscopy, documents the identity of a remarkably intriguing operation of a Ca(2+) sensor component of the ONE-GC transduction machinery, GCAP1. In the ciliary membranes, the sites of odorant transduction, GCAP1 is biochemically and physiologically coupled to ONE-GC. Strikingly, this coupling reverses its well- established function in ROS-GC1 signaling, linked with phototransduction. In response to the free Ca(2+) range from nanomolar to semimicromolar, it inhibits ROS-GC1, yet in this range, it incrementally stimulates ONE-GC. These two opposite modes of signaling two SENSORY processes by a single Ca(2+) sensor define a new transduction paradigm of membrane guanylate cyclases. This paradigm is pictorially presented.
Figures





Similar articles
-
Membrane guanylate cyclase, a multimodal transduction machine: history, present, and future directions.Front Mol Neurosci. 2014 Jul 2;7:56. doi: 10.3389/fnmol.2014.00056. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25071437 Free PMC article. Review.
-
Ca2+-modulated ONE-GC odorant signal transduction.FEBS Lett. 2009 Apr 17;583(8):1327-30. doi: 10.1016/j.febslet.2009.03.036. Epub 2009 Mar 22. FEBS Lett. 2009. PMID: 19306880
-
Distinct ONE-GC transduction modes and motifs of the odorants: Uroguanylin and CO(2).Biochem Biophys Res Commun. 2010 Jan 15;391(3):1379-84. doi: 10.1016/j.bbrc.2009.12.068. Epub 2009 Dec 22. Biochem Biophys Res Commun. 2010. PMID: 20026308 Free PMC article.
-
ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer.Biochem Biophys Res Commun. 2008 Mar 7;367(2):440-5. doi: 10.1016/j.bbrc.2007.12.153. Epub 2008 Jan 4. Biochem Biophys Res Commun. 2008. PMID: 18178149
-
Ca(2+)-modulated vision-linked ROS-GC guanylate cyclase transduction machinery.Mol Cell Biochem. 2010 Jan;334(1-2):105-15. doi: 10.1007/s11010-009-0330-z. Epub 2009 Nov 27. Mol Cell Biochem. 2010. PMID: 19943184 Review.
Cited by
-
Bicarbonate and Ca(2+) Sensing Modulators Activate Photoreceptor ROS-GC1 Synergistically.Front Mol Neurosci. 2016 Jan 28;9:5. doi: 10.3389/fnmol.2016.00005. eCollection 2016. Front Mol Neurosci. 2016. PMID: 26858600 Free PMC article.
-
Antithetical modes of and the Ca(2+) sensors targeting in ANF-RGC and ROS-GC1 membrane guanylate cyclases.Front Mol Neurosci. 2012 Apr 9;5:44. doi: 10.3389/fnmol.2012.00044. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22509151 Free PMC article.
-
Membrane guanylate cyclase, a multimodal transduction machine: history, present, and future directions.Front Mol Neurosci. 2014 Jul 2;7:56. doi: 10.3389/fnmol.2014.00056. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25071437 Free PMC article. Review.
-
Atrial natriuretic factor receptor guanylate cyclase, ANF-RGC, transduces two independent signals, ANF and Ca(2+).Front Mol Neurosci. 2014 Mar 17;7:17. doi: 10.3389/fnmol.2014.00017. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24672425 Free PMC article. Review.
-
Integrative Signaling Networks of Membrane Guanylate Cyclases: Biochemistry and Physiology.Front Mol Neurosci. 2016 Sep 15;9:83. doi: 10.3389/fnmol.2016.00083. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27695398 Free PMC article. Review.
References
-
- Munger SD, Leinders-Zufall T, Zufall F. Subsystem Organization of the mammalian sense of smell. Ann Rev Physiol. 2009;71:115–140T. - PubMed
-
- Nakamura T. Cellular and molecular constituents of olfactory sensation in vertebrates. Comp Biochem Physiol A Mol Integr Physiol. 2000;126:17–32. - PubMed
-
- Menini A. Calcium signaling and regulation in olfactory neurons. Curr Opin Neurobiol. 1999;9:419–426. - PubMed
-
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev. 1998;78:429–466. - PubMed
-
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

