Origin of light-induced spin-correlated radical pairs in cryptochrome
- PMID: 20684534
- PMCID: PMC4329313
- DOI: 10.1021/jp103401u
Origin of light-induced spin-correlated radical pairs in cryptochrome
Abstract
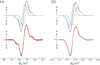
Blue-light excitation of cryptochromes and homologues uniformly triggers electron transfer (ET) from the protein surface to the flavin adenine dinucleotide (FAD) cofactor. A cascade of three conserved tryptophan residues has been considered to be critically involved in this photoreaction. If the FAD is initially in its fully oxidized (diamagnetic) redox state, light-induced ET via the tryptophan triad generates a series of short-lived spin-correlated radical pairs comprising an FAD radical and a tryptophan radical. Coupled doublet-pair species of this type have been proposed as the basis, for example, of a biological magnetic compass in migratory birds, and were found critical for some cryptochrome functions in vivo. In this contribution, a cryptochrome-like protein (CRYD) derived from Xenopus laevis has been examined as a representative system. The terminal radical-pair state FAD(•)···W324(•) of X. laevis CRYD has been characterized in detail by time-resolved electron-paramagnetic resonance (TREPR) at X-band microwave frequency (9.68 GHz) and magnetic fields around 345 mT, and at Q-band (34.08 GHz) at around 1215 mT. Different precursor states, singlet versus triplet, of radical-pair formation have been considered in spectral simulations of the experimental electron-spin polarized TREPR signals. Conclusively, we present evidence for a singlet-state precursor of FAD(•)···W324(•) radical-pair generation because at both magnetic fields, where radical pairs were studied by TREPR, net-zero electron-spin polarization has been detected. Neither a spin-polarized triplet precursor nor a triplet at thermal equilibrium can explain such an electron-spin polarization. It turns out that a two-microwave-frequency TREPR approach is essential to draw conclusions on the nature of the precursor electronic states in light-induced spin-correlated radical pair formations.
Figures



Similar articles
-
Electron transfer and spin dynamics of the radical-pair in the cryptochrome from Chlamydomonas reinhardtii by computational analysis.J Chem Phys. 2020 Feb 14;152(6):065101. doi: 10.1063/1.5133019. J Chem Phys. 2020. PMID: 32061221
-
Separation of photo-induced radical pair in cryptochrome to a functionally critical distance.Sci Rep. 2014 Jan 24;4:3845. doi: 10.1038/srep03845. Sci Rep. 2014. PMID: 24457842 Free PMC article.
-
Alternative radical pairs for cryptochrome-based magnetoreception.J R Soc Interface. 2014 Mar 26;11(95):20131063. doi: 10.1098/rsif.2013.1063. Print 2014 Jun 6. J R Soc Interface. 2014. PMID: 24671932 Free PMC article.
-
Transient EPR: using spin polarization in sequential radical pairs to study electron transfer in photosynthesis.Photosynth Res. 2009 Nov-Dec;102(2-3):335-47. doi: 10.1007/s11120-009-9411-9. Photosynth Res. 2009. PMID: 19255871 Review.
-
The Radical-Pair Mechanism of Magnetoreception.Annu Rev Biophys. 2016 Jul 5;45:299-344. doi: 10.1146/annurev-biophys-032116-094545. Epub 2016 May 16. Annu Rev Biophys. 2016. PMID: 27216936 Review.
Cited by
-
Extended Electron-Transfer in Animal Cryptochromes Mediated by a Tetrad of Aromatic Amino Acids.Biophys J. 2016 Jul 26;111(2):301-311. doi: 10.1016/j.bpj.2016.06.009. Biophys J. 2016. PMID: 27463133 Free PMC article.
-
Tracking the Electron Transfer Cascade in European Robin Cryptochrome 4 Mutants.J Am Chem Soc. 2023 May 31;145(21):11566-11578. doi: 10.1021/jacs.3c00442. Epub 2023 May 17. J Am Chem Soc. 2023. PMID: 37195086 Free PMC article.
-
The sensitivity of a radical pair compass magnetoreceptor can be significantly amplified by radical scavengers.Sci Rep. 2017 Sep 14;7(1):11640. doi: 10.1038/s41598-017-09914-7. Sci Rep. 2017. PMID: 28912470 Free PMC article.
-
Magnetic field effects in flavoproteins and related systems.Interface Focus. 2013 Oct 6;3(5):20130037. doi: 10.1098/rsfs.2013.0037. Interface Focus. 2013. PMID: 24511388 Free PMC article. Review.
-
Direct observation of long-lived radical pair between flavin and guanine in single- and double-stranded DNA-oligomers.Commun Chem. 2025 Jul 9;8(1):203. doi: 10.1038/s42004-025-01596-x. Commun Chem. 2025. PMID: 40634712 Free PMC article.
References
-
- Weber S. Biochim. Biophys. Acta. 2005;1707:1–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

