Substrate affinity of photosensitizers derived from chlorophyll-a: the ABCG2 transporter affects the phototoxic response of side population stem cell-like cancer cells to photodynamic therapy
- PMID: 20684544
- PMCID: PMC3017217
- DOI: 10.1021/mp100154j
Substrate affinity of photosensitizers derived from chlorophyll-a: the ABCG2 transporter affects the phototoxic response of side population stem cell-like cancer cells to photodynamic therapy
Abstract
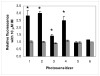
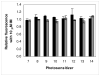
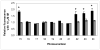
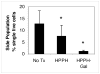
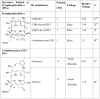
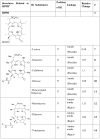
Photosensitizers (PS) synthesized with the aim of optimizing photodynamic therapy (PDT) of tumors do not always fulfill their potential when tested in vitro and in vivo in different tumor models. The ATP-dependent transporter ABCG2, a multidrug resistant pump expressed at variable levels in cancerous cells, can bind and efflux a wide range of structurally different classes of compounds including several PS used preclinically and clinically such as porphyrins and chlorins. ABCG2 may lower intracellular levels of substrate PS below the threshold for cell death in tumors treated by PDT, leaving resistant cells to repopulate the tumor. To determine some of the structural factors that affect substrate affinity of PS for ABCG2, we used an ABCG2-expressing cell line (HEK 293 482R) and its nonexpressing counterpart, and tyrosine kinase ABCG2 inhibitors in a simple flow cytometric assay to identify PS effluxed by the ABCG2 pump. We tested a series of conjugates of substrate PS with different groups attached at different positions on the tetrapyrrole macrocycle to examine whether a change in affinity for the pump occurred and whether such changes depended on the position or the structure/type of the attached group. PS without substitutions including pyropheophorbides and purpurinimides were generally substrates for ABCG2, but carbohydrate groups conjugated at positions 8, 12, 13, and 17 but not at position 3 abrogated ABCG2 affinity regardless of structure or linking moiety. At position 3, affinity was retained with the addition of iodobenzene, alkyl chains and monosaccharides, but not with disaccharides. This suggests that structural characteristics at position 3 may offer important contributions to requirements for binding to ABCG2. We examined several tumor cell lines for ABCG2 activity, and found that although some cell lines had negligible ABCG2 activity in bulk, they contained a small ABCG2-expressing side population (SP) thought to contain cells which are responsible for initiating tumor regrowth. We examined the relevance of the SP to PDT resistance with ABCG2 substrates in vitro and in vivo in the murine mammary tumor 4T1. We show for the first time in vivo that the substrate PS HPPH (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a) but not the nonsubstrate PS HPPH-Gal (a galactose conjugate of HPPH) selectively preserved the SP which was primarily responsible for regrowth in vitro. The SP could be targeted by addition of imatinib mesylate, a tyrosine kinase inhibitor which inhibits the ATPase activity of ABCG2, and prevents efflux of substrates. A PDT resistant SP may be responsible for recurrences observed both preclinically and clinically. To prevent ABCG2 mediated resistance, choosing nonsubstrate PS or administering an ABCG2 inhibitor alongside a substrate PS might be advantageous when treating ABCG2-expressing tumors with PDT.
Figures


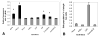



References
-
- Pandey SK, Gryshuk AL, Sajjad M, Zheng X, Chen Y, Abouzeid MM, Morgan J, Charamisinau I, Nabi HA, Oseroff A, Pandey RK. Multimodality agents for tumor imaging (PET, fluorescence) and photodynamic therapy. A possible “see and treat” approach. Journal of Medicinal Chemistry. 2005;48:6286–6295. - PubMed
-
- Li G, Slansky A, Dobhal MP, Goswami LN, Graham A, Chen Y, Kanter P, Alberico RA, Spernyak J, Morgan J, Mazurchuk R, Oseroff A, Grossman Z, Pandey RK. Chlorophyll-a analogues conjugated with aminobenzyl-DTPA as potential bifunctional agents for magnetic resonance imaging and photodynamic therapy. Bioconjug Chem. 2005;16:32–42. - PubMed
-
- Chen Y, Gryshuk A, Achilefu S, Ohulchansky T, Potter W, Zhong T, Morgan J, Chance B, Prasad PN, Henderson BW, Oseroff A, Pandey RK. A novel approach to a bifunctional photosensitizer for tumor imaging and phototherapy. Bioconjug Chem. 2005;16:1264–1274. - PubMed
-
- Pandey SK, Sajjad M, Chen Y, Zheng X, Yao R, Missert JR, Batt C, Nabi HA, Oseroff AR, Pandey RK. Comparative positron-emission tomography (PET) imaging and phototherapeutic potential of 124I-labelled Methyl 124I- Labeled Methyl- 3-(1'-m-iodobenzyloxyethyl)pyropheophorbide-a vs the Corresponding Glucose and Galactose Conjugates. J Med Chem. 2009;52:445–455. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

