Substituted E-3-(3-indolylmethylene)-1,3-dihydroindol-2-ones with antitumor activity. In depth study of the effect on growth of breast cancer cells
- PMID: 20684599
- PMCID: PMC2920599
- DOI: 10.1021/jm1007165
Substituted E-3-(3-indolylmethylene)-1,3-dihydroindol-2-ones with antitumor activity. In depth study of the effect on growth of breast cancer cells
Abstract
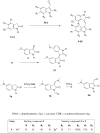
The synthesis of new substituted E-3-(3-indolylmethylene)-1,3-dihydroindol-2-ones is reported. The antitumor activity was evaluated according to protocols available at the National Cancer Institute (NCI), Bethesda, MD. Structure-activity relationships are discussed. The action of selected compounds was investigated in MCF-7 breast cancer cells. The ability of these derivatives to inhibit cellular proliferation was accompanied by increased level of p53 and its transcriptional targets p21 and Bax, interference in the cell cycle progression with cell accumulation in the G2/M phase, and activation of apoptosis.
Figures


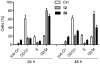

References
-
-
Potential Antitumor Agents 46. For part 45, see the following: Andreani A, Burnelli S, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Varoli L, Landi L, Prata C, Vieceli Dalla Sega F, Caliceti C, Shoemaker RH. Antitumor Activity and COMPARE Analysis of Bis-indole Derivatives. Bioorg Med Chem. doi: 10.1016/j.bmc.2010.03.063. In press.
-
-
- Andreani A, Locatelli A, Rambaldi M, Leoni A, Bossa R, Fraccari A, Galatulas I. Potential Antitumor Agents. 25. Synthesis and Cytotoxic Activity of 3-(2-Chloro-3- indolylmethylene)1,3-dihydroindol-2-ones. Anticancer Res. 1996;16:3585–3588. - PubMed
-
- Andreani A, Locatelli A, Leoni A, Morigi R, Chiericozzi M, Fraccari A, Galatulas I, Salvatore G. Synthesis and Potential Coanthracyclinic Activity of Pyridylmethylene and Indolylmethylene Lactams. Eur J Med Chem. 1998;33:905–909.
-
- Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Garaliene V. Synthesis and Antitumor Activity of 1,5,6-Substituted 3-(2-Chloro-3-indolylmethylene)1,3- dihydroindol-2-ones. J Med Chem. 2002;45:2666–2669. - PubMed
-
- Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Garaliene V, Farruggia G, Masotti L. Substituted E-3-(2-Chloro-3-indolylmethylene)1,3-dihydroindol-2- ones With Antitumor Activity. Bioorg Med Chem. 2004;12:1121–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

