Cathepsin B inhibition interferes with metastatic potential of human melanoma: an in vitro and in vivo study
- PMID: 20684763
- PMCID: PMC2925371
- DOI: 10.1186/1476-4598-9-207
Cathepsin B inhibition interferes with metastatic potential of human melanoma: an in vitro and in vivo study
Abstract
Background: Cathepsins represent a group of proteases involved in determining the metastatic potential of cancer cells. Among these are cysteinyl- (e.g. cathepsin B and cathepsin L) and aspartyl-proteases (e.g. cathepsin D), normally present inside the lysosomes as inactive proenzymes. Once released in the extracellular space, cathepsins contribute to metastatic potential by facilitating cell migration and invasiveness.
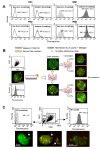
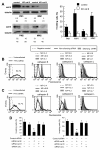
Results: In the present work we first evaluated, by in vitro procedures, the role of cathepsins B, L and D, in the remodeling, spreading and invasiveness of eight different cell lines: four primary and four metastatic melanoma cell lines. Among these, we considered two cell lines derived from a primary cutaneous melanoma and from a supraclavicular lymph node metastasis of the same patient. To this purpose, the effects of specific chemical inhibitors of these proteases, i.e. CA-074 and CA-074Me for cathepsin B, Cathepsin inhibitor II for cathepsin L, and Pepstatin A for cathepsin D, were evaluated. In addition, we also analyzed the effects of the biological inhibitors of these cathepsins, i.e. specific antibodies, on cell invasiveness. We found that i) cathepsin B, but not cathepsins L and D, was highly expressed at the surface of metastatic but not of primary melanoma cell lines and that ii) CA-074, or specific antibodies to cathepsin B, hindered metastatic cell spreading and dissemination, whereas neither chemical nor biological inhibitors of cathepsins D and L had significant effects. Accordingly, in vivo studies, i.e. in murine xenografts, demonstrated that CA-074 significantly reduced human melanoma growth and the number of artificial lung metastases.
Conclusions: These results suggest a reappraisal of the use of cathepsin B inhibitors (either chemical or biological) as innovative strategy in the management of metastatic melanoma disease.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

