Evaluation of a system of structured, pro-active care for chronic depression in primary care: a randomised controlled trial
- PMID: 20684786
- PMCID: PMC2923105
- DOI: 10.1186/1471-244X-10-61
Evaluation of a system of structured, pro-active care for chronic depression in primary care: a randomised controlled trial
Abstract
Background: People with chronic depression are frequently lost from effective care, with resulting psychological, physical and social morbidity and considerable social and financial societal costs. This randomised controlled trial will evaluate whether regular structured practice nurse reviews lead to better mental health and social outcomes for these patients and will assess the cost-effectiveness of the structured reviews compared to usual care.The hypothesis is that structured, pro-active care of patients with chronic depression in primary care will lead to a cost-effective improvement in medical and social outcomes when compared with usual general practitioner (GP) care.
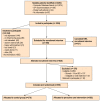
Methods/design: Participants were recruited from 42 general practices throughout the United Kingdom. Eligible participants had to have a history of chronic major depression, recurrent major depression or chronic dsythymia confirmed using the Composite International Diagnostic Interview (CIDI). They also needed to score 14 or above on the Beck Depression Inventory (BDI-II) at recruitment.Once consented, participants were randomised to treatment as usual from their general practice (controls) or the practice nurse led intervention. The intervention includes a specially prepared education booklet and a comprehensive baseline assessment of participants' mood and any associated physical and psycho-social factors, followed by regular 3 monthly reviews by the nurse over the 2 year study period. At these appointments intervention participants' mood will be reviewed, together with their current pharmacological and psychological treatments and any relevant social factors, with the nurse suggesting possible amendments according to evidence based guidelines. This is a chronic disease management model, similar to that used for other long-term conditions in primary care.The primary outcome is the BDI-II, measured at baseline and 6 monthly by self-complete postal questionnaire. Secondary outcomes collected by self-complete questionnaire at baseline and 2 years include social functioning, quality of life and data for the economic analyses. Health service data will be collected from GP notes for the 24 months before recruitment and the 24 months of the study.
Discussion: 558 participants were recruited, 282 to the intervention and 276 to the control arm. The majority were recruited via practice database searches using relevant READ codes.
Trial registration: ISRCTN36610074.
Figures
References
-
- Singleton N, Bumpstead R, O'Brien M. 'Office of National Statistics: Psychiatric morbidity among adults living in private households 2000'. London: HMSO; 2001. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources