Spatiotemporal patterns of macrophage migration inhibitory factor (Mif) expression in the mouse placenta
- PMID: 20684790
- PMCID: PMC2922212
- DOI: 10.1186/1477-7827-8-95
Spatiotemporal patterns of macrophage migration inhibitory factor (Mif) expression in the mouse placenta
Abstract
Background: Macrophage migration inhibitory factor (MIF) has special pro-inflammatory roles, affecting the functions of macrophages and lymphocytes and counter-regulating the effects of glucocorticoids on the immune response. The conspicuous expression of MIF during human implantation and early embryonic development also suggests this factor acts in reproductive functions. The overall goal of this study was to evaluate Mif expression by trophoblast and embryo placental cells during mouse pregnancy.
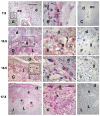
Methods: Mif was immunolocalized at implantation sites on gestation days (gd) 7.5, 10.5, 13.5 and 17.5. Ectoplacental cones and fetal placentas dissected from the maternal tissues were used for Western blotting and qRT-PCR assays on the same gestation days.
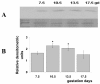
Results: During the post-implantation period (gd7.5), trophoblast giant cells showed strong Mif reactivity. In later placentation phases (gds 10.5-17.5), Mif appeared to be concentrated in the junctional zone and trophoblast giant cells. Mif protein expression increased significantly from gd7.5 to 10.5 (p = 0.005) and from gd7.5 to 13.5 (p = 0.03), remaining at high concentration as gestation proceeded. Higher mRNA expression was found on gd10.5 and was significantly different from gd13.5 (p = 0.048) and 17.5 (p = 0.009).
Conclusions: The up-regulation of Mif on gd10.5 coincides with the stage in which the placenta assumes its three-layered organization (giant cells, spongiotrophoblast and labyrinth zones), fetal blood circulation begins and population of uNK cells reaches high proportions at the maternal counter part of the placenta, suggesting that Mif may play a role in either the placentation or in the adaptation of the differentiated placenta to the uterus or still in gestational immunomodulatory responses. Moreover, it reinforces the possibility of specific activities for Mif at the maternal fetal interface.
Figures

Similar articles
-
Variation in macrophage-migration-inhibitory-factor immunoreactivity during porcine gestation.Biol Reprod. 2005 Apr;72(4):949-53. doi: 10.1095/biolreprod.104.029058. Epub 2004 Nov 24. Biol Reprod. 2005. PMID: 15564603
-
Macrophage migration inhibitory factor in fetoplacental tissues from preeclamptic pregnancies with or without fetal growth restriction.Clin Dev Immunol. 2012;2012:639342. doi: 10.1155/2012/639342. Epub 2011 Oct 4. Clin Dev Immunol. 2012. PMID: 22007254 Free PMC article.
-
Variation in macrophage migration inhibitory factor [MIF] immunoreactivity during bovine gestation.Placenta. 2012 Mar;33(3):157-63. doi: 10.1016/j.placenta.2011.12.005. Epub 2011 Dec 24. Placenta. 2012. PMID: 22200576
-
Review: putative roles for the macrophage migratory inhibitory factor at the maternal fetal interface.Placenta. 2014 Feb;35 Suppl:S51-6. doi: 10.1016/j.placenta.2013.10.015. Epub 2013 Nov 1. Placenta. 2014. PMID: 24215782 Review.
-
Macrophage migration inhibitory factor in human early pregnancy events and association with placental pathologies.Placenta. 2021 Dec;116:51-57. doi: 10.1016/j.placenta.2021.02.007. Epub 2021 Feb 15. Placenta. 2021. PMID: 33612316 Review.
Cited by
-
Stromal Cell-Derived Factor 2: A Novel Protein that Interferes in Endoplasmic Reticulum Stress Pathway in Human Placental Cells.Biol Reprod. 2016 Aug;95(2):41. doi: 10.1095/biolreprod.115.138164. Epub 2016 Jun 22. Biol Reprod. 2016. PMID: 27335075 Free PMC article.
-
Role of the Macrophage Migration Inhibitory Factor (MIF) in the survival of first trimester human placenta under induced stress conditions.Sci Rep. 2018 Aug 14;8(1):12150. doi: 10.1038/s41598-018-29797-6. Sci Rep. 2018. PMID: 30108299 Free PMC article.
-
IL-22 is expressed by the invasive trophoblast of the equine (Equus caballus) chorionic girdle.J Immunol. 2012 May 1;188(9):4181-7. doi: 10.4049/jimmunol.1103509. Epub 2012 Apr 4. J Immunol. 2012. PMID: 22490443 Free PMC article.
-
Maternal plasma and amniotic fluid chemokines screening in fetal Down syndrome.Mediators Inflamm. 2014;2014:835837. doi: 10.1155/2014/835837. Epub 2014 Nov 16. Mediators Inflamm. 2014. PMID: 25477715 Free PMC article.
-
Plasma Levels of Macrophage Migration Inhibitory Factor and d-Dopachrome Tautomerase Show a Highly Specific Profile in Early Life.Front Immunol. 2017 Jan 25;8:26. doi: 10.3389/fimmu.2017.00026. eCollection 2017. Front Immunol. 2017. PMID: 28179905 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

