Anticancer activity of a sub-fraction of dichloromethane extract of Strobilanthes crispus on human breast and prostate cancer cells in vitro
- PMID: 20684795
- PMCID: PMC2924269
- DOI: 10.1186/1472-6882-10-42
Anticancer activity of a sub-fraction of dichloromethane extract of Strobilanthes crispus on human breast and prostate cancer cells in vitro
Abstract
Background: The leaves of Strobilanthes crispus (S. crispus) which is native to the regions of Madagascar to the Malay Archipelago, are used in folk medicine for their antidiabetic, diuretic, anticancer and blood pressure lowering properties. Crude extracts of this plant have been found to be cytotoxic to human cancer cell lines and protective against chemically-induced hepatocarcinogenesis in rats. In this study, the cytotoxicity of various sub-fractions of dichloromethane extract isolated from the leaves of S. crispus was determined and the anticancer activity of one of the bioactive sub-fractions, SC/D-F9, was further analysed in breast and prostate cancer cell lines.
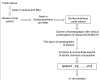
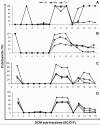
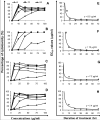
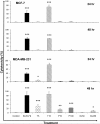
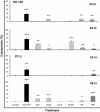
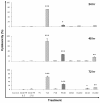
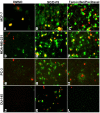
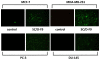
Methods: The dichloromethane extract of S. crispus was chromatographed on silica gel by flash column chromatography. The ability of the various sub-fractions obtained to induce cell death of MCF-7, MDA-MB-231, PC-3 and DU-145 cell lines was determined using the LDH assay. The dose-response effect and the EC50 values of the active sub-fraction, SC/D-F9, were determined. Apoptosis was detected using Annexin V antibody and propidium iodide staining and analysed by fluorescence microscopy and flow cytometry, while caspase 3/7 activity was detected using FLICA caspase inhibitor and analysed by fluorescence microscopy.
Results: Selected sub-fractions of the dichloromethane extract induced death of MCF-7, MDA-MB-231, PC-3 and DU-145 cells. The sub-fraction SC/D-F9, consistently killed breast and prostate cancer cell lines with low EC50 values but is non-cytotoxic to the normal breast epithelial cell line, MCF-10A. SC/D-F9 displayed relatively higher cytotoxicity compared to tamoxifen, paclitaxel, docetaxel and doxorubicin. Cell death induced by SC/D-F9 occurred via apoptosis with the involvement of caspase 3 and/or 7.
Conclusions: A dichloromethane sub-fraction of S. crispus displayed potent anticancer activities in vitro that can be further exploited for the development of a potential therapeutic anticancer agent.
Figures


Similar articles
-
Synergistic anticancer effects of a bioactive subfraction of Strobilanthes crispus and tamoxifen on MCF-7 and MDA-MB-231 human breast cancer cell lines.BMC Complement Altern Med. 2014 Jul 18;14:252. doi: 10.1186/1472-6882-14-252. BMC Complement Altern Med. 2014. PMID: 25034326 Free PMC article.
-
In vitro cytotoxicity of Strobilanthes crispus ethanol extract on hormone dependent human breast adenocarcinoma MCF-7 cell.BMC Complement Altern Med. 2012 Apr 4;12:35. doi: 10.1186/1472-6882-12-35. BMC Complement Altern Med. 2012. PMID: 22471785 Free PMC article.
-
Cell Cycle Modulation of MCF-7 and MDA-MB-231 by a Sub- Fraction of Strobilanthes crispus and its Combination with Tamoxifen.Asian Pac J Cancer Prev. 2015;16(18):8135-40. doi: 10.7314/apjcp.2015.16.18.8135. Asian Pac J Cancer Prev. 2015. PMID: 26745050
-
A review of the anticancer potential of the antimalarial herbal cryptolepis sanguinolenta and its major alkaloid cryptolepine.Ghana Med J. 2013 Sep;47(3):137-47. Ghana Med J. 2013. PMID: 24391229 Free PMC article. Review.
-
Nanoencapsulation of extracts and isolated compounds of plant origin and their cytotoxic effects on breast and cervical cancer treatments: Advantages and new challenges.Toxicon. 2024 Jun;244:107753. doi: 10.1016/j.toxicon.2024.107753. Epub 2024 May 11. Toxicon. 2024. PMID: 38740098 Review.
Cited by
-
Phytochemical profiling and anticancer potential of pistachio wastes against MCF-7 breast cancer cells: a metabolic and apoptotic pathway analysis.BMC Complement Med Ther. 2025 Jul 17;25(1):275. doi: 10.1186/s12906-025-04963-2. BMC Complement Med Ther. 2025. PMID: 40676557 Free PMC article.
-
Targeting Molecular Pathways in Breast Cancer Using Plant-Derived Bioactive Compounds: A Comprehensive Review.J Exp Pharmacol. 2025 Jun 22;17:375-401. doi: 10.2147/JEP.S528132. eCollection 2025. J Exp Pharmacol. 2025. PMID: 40585862 Free PMC article. Review.
-
Optimization of ultrasonic-assisted extraction of phenolic compounds from Clinacanthus nutans using ionic liquid (ILs) binary solvent: Application of Peleg's model and response surface methodology.PLoS One. 2025 Jul 3;20(7):e0326141. doi: 10.1371/journal.pone.0326141. eCollection 2025. PLoS One. 2025. PMID: 40608755 Free PMC article.
-
Antiproliferative and Apoptosis Induction Potential of the Methanolic Leaf Extract of Holarrhena floribunda (G. Don).Evid Based Complement Alternat Med. 2015;2015:756482. doi: 10.1155/2015/756482. Epub 2015 Mar 11. Evid Based Complement Alternat Med. 2015. PMID: 25861368 Free PMC article.
-
Regulatory Mechanism on Anti-Glycolytic and Anti-Metastatic Activities Induced by Strobilanthes crispus in Breast Cancer, In Vitro.Pharmaceuticals (Basel). 2023 Jan 20;16(2):153. doi: 10.3390/ph16020153. Pharmaceuticals (Basel). 2023. PMID: 37259304 Free PMC article.
References
-
- Fisher B, Costantino JP, Wickerham LD, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

