NF-κB inducing kinase: a key regulator in the immune system and in cancer
- PMID: 20685151
- PMCID: PMC2939163
- DOI: 10.1016/j.cytogfr.2010.06.002
NF-κB inducing kinase: a key regulator in the immune system and in cancer
Abstract
NF-κB inducing kinase (NIK) is a kinase that activates the canonical and non-canonical NF-κB pathways to control transcriptional expression of certain proteins such as cytokines, chemokines and NF-κB signaling molecules. Many advances have been made in understanding the molecular mechanisms by which the stability of NIK is regulated to affect downstream signaling. Genetic mouse models suggest that NIK plays an essential role in the regulation of the immune system as well as in the bone microenvironment. Increasing evidence links NIK to the tumorigenesis of hematological cancers, such as multiple myeloma, and solid tumors, such as pancreatic carcinoma and melanoma. Understanding the mechanism by which NIK is de-regulated will potentially provide therapeutic options for certain diseases such as autoimmunity and cancer.
Published by Elsevier Ltd.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures
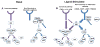


References
-
- Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–4. - PubMed
-
- Akiba H, Nakano H, Nishinaka S, Shindo M, Kobata T, Atsuta M, et al. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J Biol Chem. 1998;273:13353–8. - PubMed
-
- Smith C, Andreakos E, Crawley JB, Brennan FM, Feldmann M, Foxwell BM. NF-kappaB-inducing kinase is dispensable for activation of NF-kappaB in inflammatory settings but essential for lymphotoxin beta receptor activation of NF-kappaB in primary human fibroblasts. J Immunol. 2001;167:5895–903. - PubMed
-
- Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

