Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies
- PMID: 20685259
- PMCID: PMC2974965
- DOI: 10.1016/j.bbmt.2010.05.007
Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies
Abstract
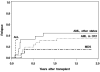
In this prospective study 60 patients of median age 46 (range: 5-60 years), with acute myelogenous leukemia (AML; n = 44), acute lymphoblastic leukemia (ALL; n = 3), or myelodysplastic syndrome (MDS; n = 13) were conditioned for allogeneic hematopoietic cell transplantation with a treosulfan/fludarabine (Flu) combination. Most patients were considered at high risk for relapse or nonrelapse mortality (NRM). Patients received intravenous treosulfan, 12 g/m(2)/day (n = 5) or 14 g/m(2)/day (n = 55) on days -6 to -4, and Flu (30 mg/m(2)/day) on days -6 to -2, followed by infusion of marrow (n = 7) or peripheral blood stem cells (n = 53) from HLA-identical siblings (n = 30) or unrelated donors (n = 30). All patients engrafted. NRM was 5% at day 100, and 8% at 2 years. With a median follow-up of 22 months, the 2-year relapse-free survival (RFS) for all patients was 58% and 88% for patients without high-risk cytogenetics. The 2-year cumulative incidence of relapse was 33% (15% for patients with MDS, 34% for AML in first remission, 50% for AML or ALL beyond first remission and 63% for AML in refractory relapse). Thus, a treosulfan/Flu regimen was well tolerated and yielded encouraging survival and disease control with minimal NRM. Further trials are warranted to compare treosulfan/Flu to other widely used regimens, and to study the impact of using this regimen in more narrowly defined groups of patients.
Copyright © 2011 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia.Biol Blood Marrow Transplant. 2014 Apr;20(4):549-55. doi: 10.1016/j.bbmt.2014.01.009. Epub 2014 Jan 16. Biol Blood Marrow Transplant. 2014. PMID: 24440648 Free PMC article. Clinical Trial.
-
Transplant Conditioning with Treosulfan/Fludarabine with or without Total Body Irradiation: A Randomized Phase II Trial in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukemia.Biol Blood Marrow Transplant. 2018 May;24(5):956-963. doi: 10.1016/j.bbmt.2017.12.785. Epub 2017 Dec 20. Biol Blood Marrow Transplant. 2018. PMID: 29274396 Clinical Trial.
-
Long-term outcome after a treosulfan-based conditioning regimen for patients with acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation.Cancer. 2017 Jul 15;123(14):2671-2679. doi: 10.1002/cncr.30646. Epub 2017 Mar 22. Cancer. 2017. PMID: 28329410
-
Missing HLA C group 1 ligand in patients with AML and MDS is associated with reduced risk of relapse and better survival after allogeneic stem cell transplantation with fludarabine and treosulfan reduced toxicity conditioning.Am J Hematol. 2017 Oct;92(10):1011-1019. doi: 10.1002/ajh.24827. Epub 2017 Jul 19. Am J Hematol. 2017. PMID: 28631269
-
Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike.Bone Marrow Transplant. 2012 Jan;47(1):5-14. doi: 10.1038/bmt.2011.88. Epub 2011 Apr 11. Bone Marrow Transplant. 2012. PMID: 21478921 Review.
Cited by
-
Conditioning with fludarabine and treosulfan compared to FLAMSA-RIC in allogeneic stem cell transplantation for myeloid malignancies: a retrospective single-center analysis.Ann Hematol. 2022 Jun;101(6):1311-1319. doi: 10.1007/s00277-022-04822-x. Epub 2022 Apr 1. Ann Hematol. 2022. PMID: 35364726 Free PMC article.
-
Long-Term Outcomes of Treosulfan- vs. Busulfan-Based Conditioning Regimen for Patients With Myelodysplastic Syndrome and Acute Myeloid Leukemia Before Hematopoietic Cell Transplantation: A Systematic Review and Meta-Analysis.Front Oncol. 2020 Dec 16;10:591363. doi: 10.3389/fonc.2020.591363. eCollection 2020. Front Oncol. 2020. PMID: 33425740 Free PMC article.
-
Determinants of Interpatient Variability in Treosulfan Pharmacokinetics in AML Patients Undergoing Autologous Stem Cell Transplantation.Int J Mol Sci. 2024 Jul 27;25(15):8215. doi: 10.3390/ijms25158215. Int J Mol Sci. 2024. PMID: 39125785 Free PMC article.
-
Hematopoietic Cell Transplantation for Older Patients with MDS.Mediterr J Hematol Infect Dis. 2014 Sep 1;6(1):e2014056. doi: 10.4084/MJHID.2014.056. eCollection 2014. Mediterr J Hematol Infect Dis. 2014. PMID: 25237469 Free PMC article. Review.
-
Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation.J Clin Oncol. 2014 Oct 10;32(29):3249-56. doi: 10.1200/JCO.2013.53.8157. Epub 2014 Aug 25. J Clin Oncol. 2014. PMID: 25154831 Free PMC article.
References
-
- Friedrichs B, Tichelli A, Bacigalupo A, et al. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol. 2010;11:331–8. - PubMed
-
- Shi-Xia X, Xian-Hua T, Hai-Qin X, Bo F, Xiang-Feng T. Total body irradiation plus cyclophosphamide versus busulphan with cyclophosphamide as conditioning regimen for patients with leukemia undergoing allogeneic stem cell transplantation: a meta-analysis. Leuk Lymphoma. 2010;51:50–60. - PubMed
-
- Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6(10):1562–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

