Synthesis and functional analysis of novel bivalent estrogens
- PMID: 20685325
- PMCID: PMC2948962
- DOI: 10.1016/j.steroids.2010.05.019
Synthesis and functional analysis of novel bivalent estrogens
Abstract
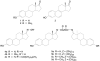
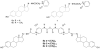
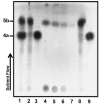
The steroid hormone estrogen plays a critical role in female development and homeostasis. Estrogen mediates its effects through binding and activation of specific estrogen receptors alpha (ERalpha) and beta (ERbeta), members of the steroid/nuclear receptor family of ligand-induced transcription factors. Due to their intimate roles in genomic and nongenomic signaling pathways, these hormones and their receptors have been also implicated in the pathologies of a variety of cancers and metabolic disorders, and have been the target of large therapeutic development efforts. The binding of estrogen to its respective receptors initiates a cascade of events that include receptor dimerization, nuclear localization, DNA binding and recruitment of co-regulatory protein complexes. In this manuscript, we investigate the potential for manipulating steroid receptor gene expression activity through the development of bivalent steroid hormones that are predicted to facilitate hormone receptor dimerization events. Data are presented for the development and testing of novel estrogen dimers, linked through their C-17 moiety, that can activate estrogen receptor alpha (ERalpha)-mediated transcription events with efficacy and potency equal to or greater than that of ERalpha's cognate ligand, 17beta-estradiol. These bivalent estrogen structures open the door to the development of a variety of steroid therapeutics that could dramatically impact future drug development in this area.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Structure activity relationships and differential interactions and functional activity of various equine estrogens mediated via estrogen receptors (ERs) ERalpha and ERbeta.Endocrinology. 2008 Oct;149(10):4857-70. doi: 10.1210/en.2008-0304. Epub 2008 Jul 3. Endocrinology. 2008. PMID: 18599548
-
Exploration of dimensions of estrogen potency: parsing ligand binding and coactivator binding affinities.J Biol Chem. 2011 Apr 15;286(15):12971-82. doi: 10.1074/jbc.M110.205112. Epub 2011 Feb 14. J Biol Chem. 2011. PMID: 21321128 Free PMC article.
-
Estrogen receptor α L543A,L544A mutation changes antagonists to agonists, correlating with the ligand binding domain dimerization associated with DNA binding activity.J Biol Chem. 2013 Jul 19;288(29):21105-21116. doi: 10.1074/jbc.M113.463455. Epub 2013 Jun 3. J Biol Chem. 2013. PMID: 23733188 Free PMC article.
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
-
Role of ERα36 in membrane-associated signaling by estrogen.Steroids. 2014 Mar;81:74-80. doi: 10.1016/j.steroids.2013.10.020. Epub 2013 Nov 16. Steroids. 2014. PMID: 24252378 Review.
Cited by
-
Towards a rational spacer design for bivalent inhibition of estrogen receptor.J Comput Aided Mol Des. 2011 Mar;25(3):253-62. doi: 10.1007/s10822-011-9417-1. Epub 2011 Feb 18. J Comput Aided Mol Des. 2011. PMID: 21331802
-
New Estrone Oxime Derivatives: Synthesis, Cytotoxic Evaluation and Docking Studies.Molecules. 2021 May 4;26(9):2687. doi: 10.3390/molecules26092687. Molecules. 2021. PMID: 34064380 Free PMC article.
-
Nonsteroidal bivalent estrogen ligands: an application of the bivalent concept to the estrogen receptor.ACS Chem Biol. 2013 Apr 19;8(4):707-15. doi: 10.1021/cb3006243. Epub 2013 Jan 30. ACS Chem Biol. 2013. PMID: 23312071 Free PMC article.
-
Recent Advances in the Use of the Dimerization Strategy as a Means to Increase the Biological Potential of Natural or Synthetic Molecules.Molecules. 2021 Apr 17;26(8):2340. doi: 10.3390/molecules26082340. Molecules. 2021. PMID: 33920597 Free PMC article. Review.
References
-
- Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–31. - PubMed
-
- Katzenellenbogen BS, Choi I, Delage-Mourroux R, et al. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74:279–85. - PubMed
-
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–5. - PubMed
-
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

