Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo
- PMID: 20685364
- PMCID: PMC2949521
- DOI: 10.1053/j.gastro.2010.05.074
Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo
Abstract
Background & aims: Hepatocyte apoptosis and activation of hepatic stellate cells (HSC) are critical events in fibrogenesis. We previously demonstrated that phagocytosis of apoptotic hepatocytes by HSC is profibrogenic. Based on this, as well as the observation that reduced nicotinamide adenine dinucleotide phosphate oxidase (NADPH) oxidase induction is central to fibrogenesis, our aim was to study the phagocytic NADPH oxidase NOX2.
Methods: An in vivo phagocytosis model was developed by injecting wild type (wt) or NOX2(-/-) mice with lentiviral-green fluorescence protein (GFP) containing a hepatocyte-specific promoter, and adeno-tumor necrosis factor-related apoptosis-inducing ligand (ad-TRAIL). Fibrosis was evaluated in bile duct ligated (BDL) wt and NOX2(-/-) mice with or without gadolinium treatment. NOX2 expression was studied in human liver samples and in HSC isolated from fibrotic livers. The fibrogenic activity of NOX2 was assessed by collagen reporter assays.
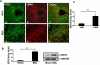
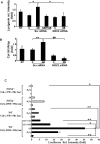
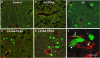
Results: In the phagocytosis model, engulfment of GFP-labeled apoptotic bodies was seen, and the expression of α-smooth muscle actin (α-SMA) and collagen I increased significantly in the wt but not in the NOX2(-/-) mice. Inhibiting apoptosis decreased the profibrogenic response. NOX2(-/-) animals exhibited significantly less fibrosis following BDL. Inactivating macrophages in wt BDL mice did not lower collagen production to the level observed in NOX2(-/-) mice, suggesting that NOX2-expressing HSC are important in fibrogenesis. NOX2 was up-regulated in HSC from fibrotic livers, and phagocytosis-induced NOX2 expression and activity were demonstrated. Based on reporter assays, production of NOX2-mediated reactive oxygen species directly induced collagen promoter activity in HSC.
Conclusions: Apoptosis and phagocytosis of hepatocytes directly induce HSC activation and initiation of fibrosis. NOX2, the phagocytic NADPH oxidase, plays a key role in this process and in liver fibrogenesis in vivo.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice.Hepatology. 2011 May;53(5):1730-41. doi: 10.1002/hep.24281. Hepatology. 2011. PMID: 21384410 Free PMC article.
-
Exogenous 8-hydroxydeoxyguanosine ameliorates liver fibrosis through the inhibition of Rac1-NADPH oxidase signaling.J Gastroenterol Hepatol. 2020 Jun;35(6):1078-1087. doi: 10.1111/jgh.14979. Epub 2020 Jan 23. J Gastroenterol Hepatol. 2020. PMID: 31907970
-
Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent.Hepatology. 2012 Dec;56(6):2316-27. doi: 10.1002/hep.25938. Hepatology. 2012. PMID: 22806357 Free PMC article.
-
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis: A review.Cell Biochem Funct. 2018 Aug;36(6):292-302. doi: 10.1002/cbf.3351. Epub 2018 Jul 20. Cell Biochem Funct. 2018. PMID: 30028028 Review.
-
Role of NADPH oxidases in liver fibrosis.Antioxid Redox Signal. 2014 Jun 10;20(17):2854-72. doi: 10.1089/ars.2013.5619. Epub 2014 Jan 24. Antioxid Redox Signal. 2014. PMID: 24040957 Free PMC article. Review.
Cited by
-
Dysregulation of redox pathways in liver fibrosis.Am J Physiol Gastrointest Liver Physiol. 2016 Oct 1;311(4):G667-G674. doi: 10.1152/ajpgi.00050.2016. Epub 2016 Aug 25. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27562057 Free PMC article. Review.
-
NADPH Oxidase Inhibition in Fibrotic Pathologies.Antioxid Redox Signal. 2020 Aug 20;33(6):455-479. doi: 10.1089/ars.2020.8032. Epub 2020 Mar 4. Antioxid Redox Signal. 2020. PMID: 32129665 Free PMC article.
-
Alcohol and HIV-Derived Hepatocyte Apoptotic Bodies Induce Hepatic Stellate Cell Activation.Biology (Basel). 2022 Jul 14;11(7):1059. doi: 10.3390/biology11071059. Biology (Basel). 2022. PMID: 36101437 Free PMC article.
-
New insights in the pathogenesis of alcohol-related liver disease: The metabolic, immunologic, and neurologic pathways☆.Liver Res. 2022 Oct 3;7(1):1-8. doi: 10.1016/j.livres.2022.09.004. eCollection 2023 Mar. Liver Res. 2022. PMID: 39959703 Free PMC article. Review.
-
Autophagy: a multifaceted partner in liver fibrosis.Biomed Res Int. 2014;2014:869390. doi: 10.1155/2014/869390. Epub 2014 Aug 31. Biomed Res Int. 2014. PMID: 25254217 Free PMC article. Review.
References
-
- Birge RB, Ucker DS. Innate apoptotic immunity: the calming touch of death. Cell Death Differ. 2008;15:1096–102. - PubMed
-
- Zhan SS, Jiang JX, Wu J, Halsted C, Friedman SL, Zern MA, Torok NJ. Phagocytosis of apoptotic bodies by hepatic stellate cells induces NADPH oxidase and is associated with liver fibrosis in vivo. Hepatology. 2006;43:435–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

