Heat shock proteins: cellular and molecular mechanisms in the central nervous system
- PMID: 20685377
- PMCID: PMC2939168
- DOI: 10.1016/j.pneurobio.2010.05.002
Heat shock proteins: cellular and molecular mechanisms in the central nervous system
Abstract
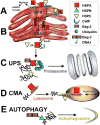
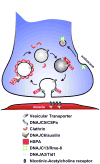
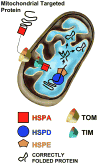
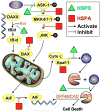
Emerging evidence indicates that heat shock proteins (HSPs) are critical regulators in normal neural physiological function as well as in cell stress responses. The functions of HSPs represent an enormous and diverse range of cellular activities, far beyond the originally identified roles in protein folding and chaperoning. HSPs are now understood to be involved in processes such as synaptic transmission, autophagy, ER stress response, protein kinase and cell death signaling. In addition, manipulation of HSPs has robust effects on the fate of cells in neurological injury and disease states. The ongoing exploration of multiple HSP superfamilies has underscored the pluripotent nature of HSPs in the cellular context, and has demanded the recent revamping of the nomenclature referring to these families to reflect a re-organization based on structure and function. In keeping with this re-organization, we first discuss the HSP superfamilies in terms of protein structure, regulation, expression and distribution in the brain. We then explore major cellular functions of HSPs that are relevant to neural physiological states, and from there we discuss known and proposed HSP impacts on major neurological disease states. This review article presents a three-part discussion on the array of HSP families relevant to neuronal tissue, their cellular functions, and the exploration of therapeutic targets of these proteins in the context of neurological diseases.
Published by Elsevier Ltd.
Figures

Similar articles
-
Heat shock and the role of the HSPs during neural plate induction in early mammalian CNS and brain development.Cell Mol Life Sci. 1997 Feb;53(2):198-211. doi: 10.1007/pl00000592. Cell Mol Life Sci. 1997. PMID: 9118008 Free PMC article. Review.
-
Heat shock proteins and exercise: a primer.Appl Physiol Nutr Metab. 2008 Oct;33(5):1050-65. doi: 10.1139/H08-069. Appl Physiol Nutr Metab. 2008. PMID: 18923583 Review.
-
Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology.Annu Rev Physiol. 1999;61:243-82. doi: 10.1146/annurev.physiol.61.1.243. Annu Rev Physiol. 1999. PMID: 10099689 Review.
-
The heat shock response: life on the verge of death.Mol Cell. 2010 Oct 22;40(2):253-66. doi: 10.1016/j.molcel.2010.10.006. Mol Cell. 2010. PMID: 20965420 Review.
-
Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets.Inflamm Allergy Drug Targets. 2007 Jun;6(2):91-100. doi: 10.2174/187152807780832274. Inflamm Allergy Drug Targets. 2007. PMID: 17692032 Review.
Cited by
-
Heat stress and immune response phenotype affect DNA methylation in blood mononuclear cells from Holstein dairy cows.Sci Rep. 2021 May 31;11(1):11371. doi: 10.1038/s41598-021-89951-5. Sci Rep. 2021. PMID: 34059695 Free PMC article.
-
Does sauna bathing protect against dementia?Prev Med Rep. 2020 Oct 2;20:101221. doi: 10.1016/j.pmedr.2020.101221. eCollection 2020 Dec. Prev Med Rep. 2020. PMID: 33088678 Free PMC article.
-
Upregulation and phosphorylation of HspB1/Hsp25 and HspB5/αB-crystallin after transient middle cerebral artery occlusion in rats.Cell Stress Chaperones. 2017 Jul;22(4):653-663. doi: 10.1007/s12192-017-0794-9. Epub 2017 Apr 20. Cell Stress Chaperones. 2017. PMID: 28425051 Free PMC article.
-
Elevated Expression of HSP72 in the Prefrontal Cortex and Hippocampus of Rats Subjected to Chronic Mild Stress and Treated with Imipramine.Int J Mol Sci. 2023 Dec 23;25(1):243. doi: 10.3390/ijms25010243. Int J Mol Sci. 2023. PMID: 38203414 Free PMC article.
-
Exploring Evolutionary Adaptations and Genomic Advancements to Improve Heat Tolerance in Chickens.Animals (Basel). 2024 Jul 30;14(15):2215. doi: 10.3390/ani14152215. Animals (Basel). 2024. PMID: 39123741 Free PMC article. Review.
References
-
- Ackerley S, James PA, Kalli A, French S, Davies KE, Talbot K. A mutation in the small heat-shock protein HSPB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum Mol Genet. 2006;15:347–354. - PubMed
-
- Akbar MT, Lundberg AM, Liu K, Vidyadaran S, Wells KE, Dolatshad H, Wynn S, Wells DJ, Latchman DS, de Belleroche J. The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem. 2003;278:19956–19965. - PubMed
-
- Akbar MT, Wells DJ, Latchman DS, de Belleroche J. Heat shock protein 27 shows a distinctive widespread spatial and temporal pattern of induction in CNS glial and neuronal cells compared to heat shock protein 70 and caspase 3 following kainate administration. Brain Res Mol Brain Res. 2001;93:148–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

