Cardiac muscle ring finger-1--friend or foe?
- PMID: 20685572
- PMCID: PMC2917387
- DOI: 10.1016/j.tcm.2010.03.001
Cardiac muscle ring finger-1--friend or foe?
Abstract
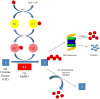
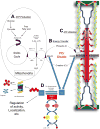
The ubiquitin proteasome system plays a role in regulating protein activity and is integral to the turnover of damaged and worn proteins. In this review, we discuss the recently described relationship between the ubiquitin proteasome system and the cardiac creatine kinase/phosphocreatine shuttle, an essential component of adenosine triphosphate generation and energy shuttling within the heart. The ubiquitin ligase muscle ring finger-1 (MuRF1) binds creatine kinase, leading to its ubiquitination and possible degradation. Muscle ring finger-1 may also be integral in the regulation of creatine kinase activity in vivo. Because there is a close relationship between the cardiac creatine kinase/phosphocreatine shuttle activity and heart failure, these findings suggest that MuRF1's role in protein quality control of creatine kinase may be vital to the regulation and maintenance of cardiac energetics to protect against heart failure.
(c) 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Adams V, Linke A, Gielen S, et al. Modulation of Murf-1 and MAFbx expression in the myocardium by physical exercise training. Eur J Cardiovasc Prev Rehabil. 2008;15:293–299. - PubMed
-
- Boehm E, Ventura-Clapier R, Mateo P, et al. Glycolysis supports calcium uptake by the sarcoplasmic reticulum in skinned ventricular fibres of mice deficient in mitochondrial and cytosolic creatine kinase. J Mol Cell Cardiol. 2000;32:891–902. - PubMed
-
- Dieni CA, Storey KB. Creatine kinase regulation by reversible phosphorylation in frog muscle. Comp Biochem Physiol B Biochem Mol Biol. 2009;152:405–412. - PubMed
-
- Dunlop RA, Brunk UT, Rodgers KJ. Oxidized proteins: Mechanisms of removal and consequences of accumulation. IUBMB Life. 2009;61:522–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

