Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor beta
- PMID: 20685654
- PMCID: PMC2952202
- DOI: 10.1074/jbc.M110.127183
Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor beta
Abstract
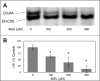
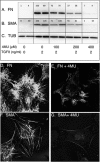
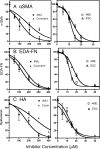
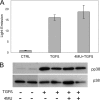
TGFβ induces fibrosis in healing corneal wounds, and in vitro corneal keratocytes up-regulate expression of several fibrosis-related genes in response to TGFβ. Hyaluronan (HA) accumulates in healing corneas, and HA synthesis is induced by TGFβ by up-regulation of HA synthase 2. This study tested the hypothesis that HA acts as an extracellular messenger, enhancing specific fibrotic responses of keratocytes to TGFβ. HA synthesis inhibitor 4-methylumbelliferone (4MU) blocked TGFβ induction of HA synthesis in a concentration-dependent manner. 4MU also inhibited TGFβ-induced up-regulation of α-smooth muscle actin, collagen type III, and extra domain A-fibronectin. Chemical analogs of 4MU also inhibited fibrogenic responses in proportion to their inhibition of HA synthesis. 4MU, however, showed no effect on TGFβ induction of luciferase by the 3TP-Lux reporter plasmid. Inhibition of HA using siRNA to HA synthase 2 reduced TGFβ up-regulation of smooth muscle actin, fibronectin, and cell division. Similarly, brief treatment of keratocytes with hyaluronidase reduced TGFβ responses. These results suggest that newly synthesized cell-associated HA acts as an extracellular enhancer of wound healing and fibrosis in keratocytes by augmenting a limited subset of the cellular responses to TGFβ.
Figures



Similar articles
-
A rapid transient increase in hyaluronan synthase-2 mRNA initiates secretion of hyaluronan by corneal keratocytes in response to transforming growth factor beta.J Biol Chem. 2007 Apr 27;282(17):12475-83. doi: 10.1074/jbc.M609280200. Epub 2007 Feb 27. J Biol Chem. 2007. PMID: 17327235 Free PMC article.
-
Efficient TGFβ-induced epithelial-mesenchymal transition depends on hyaluronan synthase HAS2.Oncogene. 2013 Sep 12;32(37):4355-65. doi: 10.1038/onc.2012.475. Epub 2012 Oct 29. Oncogene. 2013. PMID: 23108409 Free PMC article.
-
TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signaling.Exp Eye Res. 2002 Dec;75(6):645-57. doi: 10.1006/exer.2002.2066. Exp Eye Res. 2002. PMID: 12470966
-
Role of transforming growth factor Beta in corneal function, biology and pathology.Curr Mol Med. 2010 Aug;10(6):565-78. doi: 10.2174/1566524011009060565. Curr Mol Med. 2010. PMID: 20642439 Free PMC article. Review.
-
Hyaluronan: More than just a wrinkle filler.Glycobiology. 2016 Jun;26(6):553-9. doi: 10.1093/glycob/cww033. Epub 2016 Mar 9. Glycobiology. 2016. PMID: 26964566 Free PMC article. Review.
Cited by
-
Provisional matrix: A role for versican and hyaluronan.Matrix Biol. 2017 Jul;60-61:38-56. doi: 10.1016/j.matbio.2016.12.001. Epub 2016 Dec 6. Matrix Biol. 2017. PMID: 27932299 Free PMC article. Review.
-
Compressed Collagen Enhances Stem Cell Therapy for Corneal Scarring.Stem Cells Transl Med. 2018 Jun;7(6):487-494. doi: 10.1002/sctm.17-0258. Epub 2018 Apr 14. Stem Cells Transl Med. 2018. PMID: 29654654 Free PMC article.
-
Inhibition of NF-kappaB with Dehydroxymethylepoxyquinomicin modifies the function of human peritoneal mesothelial cells.Am J Transl Res. 2016 Dec 15;8(12):5756-5765. eCollection 2016. Am J Transl Res. 2016. PMID: 28078047 Free PMC article.
-
Proteoglycans in Mechanobiology of Tissues and Organs: Normal Functions and Mechanopathology.Proteoglycan Res. 2024 Apr-Jun;2(2):e21. doi: 10.1002/pgr2.21. Epub 2024 May 20. Proteoglycan Res. 2024. PMID: 39584146 Free PMC article.
-
Corneal Opacity: Cell Biological Determinants of the Transition From Transparency to Transient Haze to Scarring Fibrosis, and Resolution, After Injury.Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):22. doi: 10.1167/iovs.63.1.22. Invest Ophthalmol Vis Sci. 2022. PMID: 35044454 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

