Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups
- PMID: 20685690
- PMCID: PMC4351376
- DOI: 10.1093/hmg/ddq325
Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups
Abstract
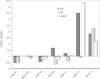
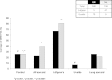
The HLA class II (DRB1 and DQB1) associations with sarcoidosis have been studied by several groups but often without consistent results. In this paper, we consider the hypothesis that observed inconsistencies relate to distinct, genetically encoded disease phenotypes which differ in prevalence between centres. We therefore typed HLA-DRB1 and DQB1 in 340 UK, 139 Dutch and 163 Japanese sarcoidosis patients and, respectively, 354, 218 and 168 healthy controls from these populations. We applied consistent phenotyping and genotyping and investigated associations between HLA class II alleles and distinct disease phenotypes within and between ethnic groups. DRB1*01 and DQB1*0501 are protective against all manifestations of sarcoidosis. Lung-predominant sarcoidosis is associated with DRB1*12 and *14. Löfgren's syndrome is a common sarcoidosis phenotype in the Dutch and is strongly associated with the DRB1*0301 allele. This phenotype is not seen among the Japanese in whom DRB1*0301 is absent. The same allele is protective for UK uveitis. Sarcoid uveitis is common in Japan. The DRB1*04-DQB1*0301 haplotype is a risk factor for this disease manifestation in Japanese and UK subjects but protective for sarcoidosis overall. We show that distinct sarcoidosis phenotypes have similar genetic associations across ethnic groups. The disease case mix differs between centres and may be explained by different ethnic allelic frequencies.
Figures


References
-
- Hillerdal G., Nou E., Osterman K., Schmekel B. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am. Rev. Respir. Dis. 1984;130:29–32. - PubMed
-
- Costabel U., Hunninghake G.W. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur. Respir. J. 1999;14:735–737. doi: 10.1034/j.1399-3003.1999.14d02.x. - DOI - PubMed
-
- Schurmann M., Lympany P.A., Reichel P., Muller-Myhsok B., Wurm K., Schlaak M., Muller-Quernheim J., du Bois R.M., Schwinger E. Familial sarcoidosis is linked to the major histocompatibility complex region. Am. J. Respir. Crit. Care. Med. 2000;162:861–864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

