The influence of tetrad shape and intersporal callose wall formation on pollen aperture pattern ontogeny in two eudicot species
- PMID: 20685726
- PMCID: PMC2944975
- DOI: 10.1093/aob/mcq152
The influence of tetrad shape and intersporal callose wall formation on pollen aperture pattern ontogeny in two eudicot species
Abstract
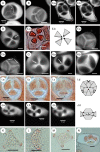
Background and aims: In flowering plants, microsporogenesis is accompanied by various types of cytoplasmic partitioning (cytokinesis). Patterns of male cytokinesis are suspected to play a role in the diversity of aperture patterns found in pollen grains of angiosperms. The relationships between intersporal wall formation, tetrad shape and pollen aperture pattern ontogeny are studied.
Methods: A comparative analysis of meiosis and aperture distribution was performed within tetrads in two triporate eudicot species with contrasting aperture arrangements within their tetrads [Epilobium roseum (Onagraceae) and Paranomus reflexus (Proteaceae)].
Key results and conclusions: Intersporal wall formation is a two-step process in both species. Cytokinesis is first achieved by the formation of naked centripetal cell plates. These naked cell plates are then covered by additional thick, localized callose deposits that differ in location between the two species. Apertures are finally formed in areas in which additional callose is deposited on the cell plates. The recorded variation in tetrad shape is correlated with variations in aperture pattern, demonstrating the role of cell partitioning in aperture pattern ontogeny.
Figures
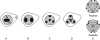

References
-
- Albert B, Gouyon P-H, Ressayre A. Microsporogenesis variation in Codiaeum producing inaperturate pollen grain. Comptes rendus biologies. 2009;332:507–516. - PubMed
-
- Albert B, Matamoro-Vidal A, Raquin C, Nadot S. Formation and function of a new pollen aperture pattern in angiosperms: the proximal sulcus of Tillandsia leiboldiana (Bromeliaceae) American Journal of Botany. 2010;97:365–368. - PubMed
-
- Albertsen MC, Palmer RG. A comparative light- and electron-microscopic study of microsporogenesis in male sterile (MS1) and male fertile soybeans (Glycine max (L.) Merr.) American Journal of Botany. 1979;66:253–265.
-
- Arens K. Prova de calose por meio da microscopia a luz fluorescente e aplicações do metodo. Lilloa. 1949;18:71–75.
-
- Bandhari NN. The microsporangium. In: Johri BM, editor. The embryology of angiosperms. Berlin: Springer Verlag; 1984. pp. 71–80.

