NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts
- PMID: 20685750
- PMCID: PMC3004009
- DOI: 10.1136/thx.2009.113456
NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts
Abstract
Background: Persistence of myofibroblasts is believed to contribute to the development of fibrosis in idiopathic pulmonary fibrosis (IPF). Transforming growth factor beta1 (TGFbeta1) irreversibly converts fibroblasts into pathological myofibroblasts, which express smooth muscle alpha-actin (alpha-SMA) and produce extracellular matrix proteins, such as procollagen I (alpha1). Reactive oxygen species produced by NADPH oxidases (NOXs) have been shown to regulate cell differentiation. It was hypothesised that NOX could be expressed in parenchymal pulmonary fibroblasts and could mediate TGFbeta1-stimulated conversion of fibroblasts into myofibroblasts.
Methods: Fibroblasts were cultured from the lung of nine controls and eight patients with IPF. NOX4, alpha-SMA and procollagen I (alpha1) mRNA and protein expression, reactive oxygen species production and Smad2/3 phosphorylation were quantified, in the absence and in the presence of incubation with TGFbeta1. Migration of platelet-derived growth factor (PDGF)-induced fibroblasts was also assessed.
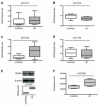
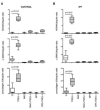
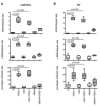
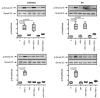
Results: It was found that (1) NOX4 mRNA and protein expression was upregulated in pulmonary fibroblasts from patients with IPF and correlated with mRNA expression of alpha-SMA and procollagen I (alpha1) mRNA; (2) TGFbeta1 upregulated NOX4, alpha-SMA and procollagen I (alpha1) expression in control and IPF fibroblasts; (3) the change in alpha-SMA and procollagen I (alpha1) expression in response to TGFbeta1 was inhibited by antioxidants and by a NOX4 small interfering RNA (siRNA); (4) NOX4 modulated alpha-SMA and procollagen I (alpha1) expression by controlling activation of Smad2/3; and (5) NOX4 modulated PDGF-induced fibroblast migration.
Conclusion: NOX4 is critical for modulation of the pulmonary myofibroblast phenotype in IPF, probably by modulating the response to TGFbeta1 and PDGF.
Conflict of interest statement
Figures


Similar articles
-
TGF-β1 stimulates HDAC4 nucleus-to-cytoplasm translocation and NADPH oxidase 4-derived reactive oxygen species in normal human lung fibroblasts.Am J Physiol Lung Cell Mol Physiol. 2017 Jun 1;312(6):L936-L944. doi: 10.1152/ajplung.00256.2016. Epub 2017 Mar 23. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28336812 Free PMC article.
-
NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts.Circ Res. 2005 Oct 28;97(9):900-7. doi: 10.1161/01.RES.0000187457.24338.3D. Epub 2005 Sep 22. Circ Res. 2005. PMID: 16179589
-
Transforming growth factor β1 (TGFβ1)-induced CD44V6-NOX4 signaling in pathogenesis of idiopathic pulmonary fibrosis.J Biol Chem. 2017 Jun 23;292(25):10490-10519. doi: 10.1074/jbc.M116.752469. Epub 2017 Apr 7. J Biol Chem. 2017. PMID: 28389561 Free PMC article.
-
Signalling pathways from NADPH oxidase-4 to idiopathic pulmonary fibrosis.Int J Biochem Cell Biol. 2011 Aug;43(8):1086-9. doi: 10.1016/j.biocel.2011.04.003. Epub 2011 Apr 12. Int J Biochem Cell Biol. 2011. PMID: 21513813 Review.
-
Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases.Kidney Int. 2011 May;79(9):944-56. doi: 10.1038/ki.2010.516. Epub 2011 Feb 9. Kidney Int. 2011. PMID: 21307839 Free PMC article. Review.
Cited by
-
The Effect of Physalis angulata L. Administration on Gene Expressions Related to Lung Fibrosis Resolution in Mice-Induced Bleomycin.J Exp Pharmacol. 2024 Feb 1;16:49-60. doi: 10.2147/JEP.S439932. eCollection 2024. J Exp Pharmacol. 2024. PMID: 38317831 Free PMC article.
-
From form to function: the role of Nox4 in the cardiovascular system.Front Physiol. 2012 Nov 1;3:412. doi: 10.3389/fphys.2012.00412. eCollection 2012. Front Physiol. 2012. PMID: 23125837 Free PMC article.
-
Nox2-Mediated PI3K and Cofilin Activation Confers Alternate Redox Control of Macrophage Pinocytosis.Antioxid Redox Signal. 2017 Jun 1;26(16):902-916. doi: 10.1089/ars.2016.6639. Epub 2016 Sep 13. Antioxid Redox Signal. 2017. PMID: 27488058 Free PMC article.
-
Lung extracellular matrix and redox regulation.Redox Biol. 2016 Aug;8:305-15. doi: 10.1016/j.redox.2016.02.005. Epub 2016 Feb 18. Redox Biol. 2016. PMID: 26938939 Free PMC article. Review.
-
Reduced Glutathione Level Promotes Epithelial-Mesenchymal Transition in Lens Epithelial Cells via a Wnt/β-Catenin-Mediated Pathway: Relevance for Cataract Therapy.Am J Pathol. 2017 Nov;187(11):2399-2412. doi: 10.1016/j.ajpath.2017.07.018. Epub 2017 Aug 19. Am J Pathol. 2017. PMID: 28827139 Free PMC article.
References
-
- Dempsey OJ. Clinical review: Idiopathic pulmonary fibrosis--past, present and future. Respir Med. 2006;100:1871–1885. - PubMed
-
- Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: Multiple causes and multiple mechanisms? Eur Respir J. 2007;30:835–839. - PubMed
-
- Zhang K, Gharaee-Kermani M, McGarry B, et al. In situ hybridization analysis of rat lung alpha 1(i) and alpha 2(i) collagen gene expression in pulmonary fibrosis induced by endotracheal bleomycin injection. Lab Invest. 1994;70:192–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
