Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1
- PMID: 20685844
- PMCID: PMC2926749
- DOI: 10.1101/cshperspect.a003004
Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1
Abstract
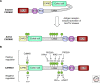
The signaling pathway controlling antigen receptor-induced regulation of the transcription factor NF-kappaB plays a key role in lymphocyte activation and development and the generation of lymphomas. Work of the past decade has led to dramatic progress in the identification and characterization of new players in the pathway. Moreover, novel enzymatic activities relevant for this pathway have been discovered, which represent interesting drug targets for immuno-suppression or lymphoma treatment. Here, we summarize these findings and give an outlook on interesting open issues that need to be addressed in the future.
Figures



Similar articles
-
The CARD11-BCL10-MALT1 (CBM) signalosome complex: Stepping into the limelight of human primary immunodeficiency.J Allergy Clin Immunol. 2014 Aug;134(2):276-84. doi: 10.1016/j.jaci.2014.06.015. J Allergy Clin Immunol. 2014. PMID: 25087226 Free PMC article. Review.
-
The CBM signalosome: potential therapeutic target for aggressive lymphoma?Cytokine Growth Factor Rev. 2014 Apr;25(2):175-83. doi: 10.1016/j.cytogfr.2013.12.008. Epub 2013 Dec 24. Cytokine Growth Factor Rev. 2014. PMID: 24411492 Free PMC article. Review.
-
The Ca2+-dependent phosphatase calcineurin controls the formation of the Carma1-Bcl10-Malt1 complex during T cell receptor-induced NF-kappaB activation.J Biol Chem. 2011 Mar 4;286(9):7522-34. doi: 10.1074/jbc.M110.155895. Epub 2011 Jan 3. J Biol Chem. 2011. PMID: 21199863 Free PMC article.
-
Bcl10 is phosphorylated on Ser138 by Ca2+/calmodulin-dependent protein kinase II.Mol Immunol. 2007 Mar;44(8):2095-100. doi: 10.1016/j.molimm.2006.09.012. Epub 2006 Oct 18. Mol Immunol. 2007. PMID: 17052756
-
MALT1--a universal soldier: multiple strategies to ensure NF-κB activation and target gene expression.FEBS J. 2015 Sep;282(17):3286-97. doi: 10.1111/febs.13325. Epub 2015 Jun 10. FEBS J. 2015. PMID: 25996250 Review.
Cited by
-
Combined immunodeficiency due to MALT1 mutations, treated by hematopoietic cell transplantation.J Clin Immunol. 2015 Feb;35(2):135-46. doi: 10.1007/s10875-014-0125-1. Epub 2015 Jan 28. J Clin Immunol. 2015. PMID: 25627829 Free PMC article.
-
Expanding spectrum, intrafamilial diversity, and therapeutic challenges from 15 patients with heterozygous CARD11-associated diseases: A single center experience.Front Immunol. 2022 Nov 3;13:1020927. doi: 10.3389/fimmu.2022.1020927. eCollection 2022. Front Immunol. 2022. PMID: 36405754 Free PMC article.
-
Bioinformatics Analysis of miRNAs Targeting TRAF5 in DLBCL Involving in NF-κB Signaling Pathway and Affecting the Apoptosis and Signal Transduction.Genet Res (Camb). 2022 Dec 23;2022:3222253. doi: 10.1155/2022/3222253. eCollection 2022. Genet Res (Camb). 2022. PMID: 36619898 Free PMC article.
-
Phosphorylation of Carma1, but not Bcl10, by Akt regulates TCR/CD28-mediated NF-κB induction and cytokine production.Mol Immunol. 2014 May;59(1):110-6. doi: 10.1016/j.molimm.2014.01.011. Epub 2014 Feb 16. Mol Immunol. 2014. PMID: 24548923 Free PMC article.
-
CARMA1 is necessary for optimal T cell responses in a murine model of allergic asthma.J Immunol. 2011 Dec 15;187(12):6197-207. doi: 10.4049/jimmunol.1101348. Epub 2011 Nov 9. J Immunol. 2011. PMID: 22075698 Free PMC article.
References
-
- Bertin J, Wang L, Guo Y, Jacobson MD, Poyet JL, Srinivasula SM, Merriam S, DiStefano PS, Alnemri ES 2001. CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane- associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-kappa B. J Biol Chem 276: 11877–11882 - PubMed
-
- Bonizzi G, Karin M 2004. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25: 280–288 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
