The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival
- PMID: 20685850
- PMCID: PMC2954639
- DOI: 10.1210/me.2010-0201
The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival
Abstract
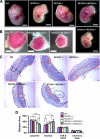
Nuclear receptor coactivator 1 [NCOA1/steroid receptor coactivator (SRC)-1] and NCOA3 (SRC-3/AIB1/ACTR) constitute two thirds of the SRC (steroid receptor coactivator) family. Although in vitro experiments have suggested overlapping functions between NCOA1 and NCOA3, their in vivo functional relationship is poorly understood. In this study, NCOA1 and NCOA3 double knockout mice were generated to determine the compensatory roles of NCOA1 and NCOA3 in development. NCOA1(-/-) mice survived normally, whereas most NCOA3(-/-) embryos were viable at embryonic d 13.5 (E13.5). In contrast, the majority of double-knockout (DKO) embryos died by E13.5. NCOA1 and NCOA3 are expressed in the labyrinth, and labyrinths of NCOA1(+/-);NCOA3(-/-) and DKO placentas were small compared with wild-type and single-knockout labyrinths. DKO labyrinths exhibited low densities of maternal blood sinuses and fetal capillaries and displayed fetomaternal blood transfusion. At the interface between maternal and fetal circulations, layer I sinusoidal trophoblast giant cells showed a reduced density of microvilli. Layer III syncytiotrophoblasts appeared to accumulate large lipid droplets and have reduced density and deepened invaginations of the intrasyncytial bays. The endothelial layer in DKO labyrinth showed abnormal morphologies and had large lipid droplets. Furthermore, disruption of NCOA1 and NCOA3 increased labyrinth trophoblast proliferation and their progenitor gene expression but decreased their differentiation gene expression. NCOA1 and NCOA3 deficiencies also affected the expression of several genes for placental morphogenesis including TGFβ-, peroxisome proliferator-activated receptor-β-, and peroxisome proliferator-activated receptor-γ-regulated genes and for glucose transportation including GLUT1 and Cx26. These findings demonstrate that NCOA1 and NCOA3 cooperatively regulate placental morphogenesis and embryo survival.
Figures






References
-
- Xu J, Li Q 2003 Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol 17:1681–1692 - PubMed
-
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW 1995 Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 - PubMed
-
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS 1997 AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

