Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens
- PMID: 20685851
- PMCID: PMC2954638
- DOI: 10.1210/me.2010-0189
Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens
Abstract
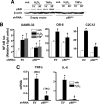
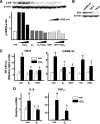
Aging or acute loss of estrogens or androgens increases the levels of reactive oxygen species, activates nuclear factor-κB (NF-κB), and promotes the phosphorylation of p66(shc), a redox enzyme that amplifies mitochondrial reactive oxygen species generation and stimulates apoptosis. We report that in mesenchymal progenitor and osteoblastic cell models, H(2)O(2) activated a protein kinase C (PKC)β/p66(shc)/NF-κB signaling cascade and that p66(shc) was an essential mediator of the stimulating effects of H(2)O(2) on the apoptosis of osteoblastic cells as well as their ability to activate NF-κB. 17β-Estradiol (E(2)) or the nonaromatizable androgen dihydrotestosterone abrogated the effects of H(2)O(2) on p66(shc) and NF-κB activation by attenuating the phosphorylation of the redox-sensitive cytoplasmic kinase PKCβ. Additionally, both E(2) and dihydrotestosterone prevented H(2)O(2)-induced apoptosis by a mechanism that involved attenuation of p66(shc) resulting from decreased phosphorylation of PKCβ. Consistent with a kinase-mediated mechanism of sex steroid action, the effects of E(2) were reproduced by a polymeric form of estradiol that is not capable of stimulating the nuclear-initiated actions of ERα. These results demonstrate that p66(shc) is an essential mediator of the effects of oxidative stress on osteoblastic cell apoptosis, NF-κB activation, and cytokine production. The ability of either estrogen or androgen to attenuate the effects of oxidative stress on osteoblastic cell apoptosis, NF-κB activation, and cytokine production results from their common property to suppress PKCβ-induced p66(shc) phosphorylation via a mechanism that does not require stimulation of the nuclear-initiated actions of sex steroids.
Figures


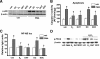
Similar articles
-
Glucocorticoids and tumor necrosis factor α increase oxidative stress and suppress Wnt protein signaling in osteoblasts.J Biol Chem. 2011 Dec 30;286(52):44326-35. doi: 10.1074/jbc.M111.283481. Epub 2011 Oct 26. J Biol Chem. 2011. PMID: 22030390 Free PMC article.
-
D,L-sulforaphane-induced apoptosis in human breast cancer cells is regulated by the adapter protein p66Shc.J Cell Biochem. 2012 Feb;113(2):599-610. doi: 10.1002/jcb.23386. J Cell Biochem. 2012. PMID: 21956685 Free PMC article.
-
Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERalpha.J Bone Miner Res. 2010 Apr;25(4):769-81. doi: 10.1359/jbmr.091017. J Bone Miner Res. 2010. PMID: 19821774 Free PMC article.
-
p66(Shc) protein, oxidative stress, and cardiovascular complications of diabetes: the missing link.J Mol Med (Berl). 2009 Sep;87(9):885-91. doi: 10.1007/s00109-009-0499-3. Epub 2009 Jul 10. J Mol Med (Berl). 2009. PMID: 19590843 Review.
-
p66(Shc): at the crossroad of oxidative stress and the genetics of aging.Trends Mol Med. 2003 May;9(5):206-10. doi: 10.1016/s1471-4914(03)00048-0. Trends Mol Med. 2003. PMID: 12763525 Review.
Cited by
-
Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects.Nanomaterials (Basel). 2019 Dec 24;10(1):50. doi: 10.3390/nano10010050. Nanomaterials (Basel). 2019. PMID: 31878270 Free PMC article.
-
Protective Action of Neurotrophic Factors and Estrogen against Oxidative Stress-Mediated Neurodegeneration.J Toxicol. 2011;2011:405194. doi: 10.1155/2011/405194. Epub 2011 May 31. J Toxicol. 2011. PMID: 21776259 Free PMC article.
-
Extra-nuclear effects of estrogen on cortical bone in males require ERαAF-1.J Mol Endocrinol. 2017 Feb;58(2):105-111. doi: 10.1530/JME-16-0209. Epub 2017 Jan 5. J Mol Endocrinol. 2017. PMID: 28057769 Free PMC article.
-
Emerging role of protein kinase C in energy homeostasis: A brief overview.World J Diabetes. 2014 Jun 15;5(3):385-92. doi: 10.4239/wjd.v5.i3.385. World J Diabetes. 2014. PMID: 24936260 Free PMC article. Review.
-
Ultrastructural Evidence of Mitochondrial Dysfunction in Osteomyelitis Patients.Int J Mol Sci. 2023 Mar 16;24(6):5709. doi: 10.3390/ijms24065709. Int J Mol Sci. 2023. PMID: 36982790 Free PMC article.
References
-
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC 2007 Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282:27285–27297 - PMC - PubMed
-
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG 2005 Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233 - PubMed
-
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG 1999 The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313 - PubMed
-
- Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM 2006 Redox-sensitive kinases of the nuclear factor-κB signaling pathway. Antioxid Redox Signal 8:1791–1806 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

