Conformation of the mineralocorticoid receptor N-terminal domain: evidence for induced and stable structure
- PMID: 20685853
- PMCID: PMC5417395
- DOI: 10.1210/me.2010-0005
Conformation of the mineralocorticoid receptor N-terminal domain: evidence for induced and stable structure
Abstract
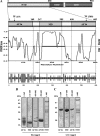
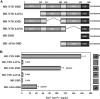
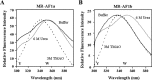
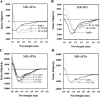
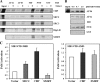
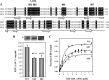
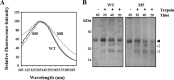
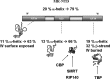
The mineralocorticoid receptor (MR) binds the steroid hormones aldosterone and cortisol and has an important physiological role in the control of salt homeostasis. Regions of the protein important for gene regulation have been mapped to the amino-terminal domain (NTD) and termed activation function (AF)1a, AF1b, and middle domain (MD). In the present study, we used a combination of biophysical and biochemical techniques to investigate the folding and function of the MR-NTD transactivation functions. We demonstrate that MR-AF1a and MR-MD have relatively little stable secondary structure but have the propensity to form α-helical conformation. Induced folding of the MR-MD enhanced protein-protein binding with a number of coregulatory proteins, including the coactivator cAMP response element-binding protein-binding protein and the corepressors SMRT and RIP140. By contrast, the MR-AF1b domain appeared to have a more stable conformation consisting predominantly of β-secondary structure. Furthermore, MR-AF1b specifically interacted with the TATA-binding protein, via an LxxLL-like motif, in the absence of induced folding. Together, these data suggest that the MR-NTD contains a complex transactivation system made up of distinct structural and functional domains. The results are discussed in the context of the induced folding paradigm for steroid receptor NTDs.
Figures


Similar articles
-
Structural and biochemical mechanisms for the specificity of hormone binding and coactivator assembly by mineralocorticoid receptor.Mol Cell. 2005 Aug 5;19(3):367-80. doi: 10.1016/j.molcel.2005.06.026. Mol Cell. 2005. PMID: 16061183
-
Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9.J Biol Chem. 2007 Jan 19;282(3):1998-2010. doi: 10.1074/jbc.M607741200. Epub 2006 Nov 14. J Biol Chem. 2007. PMID: 17105732
-
NF-YC functions as a corepressor of agonist-bound mineralocorticoid receptor.J Biol Chem. 2010 Mar 12;285(11):8084-93. doi: 10.1074/jbc.M109.053371. Epub 2010 Jan 6. J Biol Chem. 2010. PMID: 20054001 Free PMC article.
-
[Corticosteroid hormones: mechanisms involved in the recognition of aldosterone by mineralocorticoid receptors].J Soc Biol. 1999;193(4-5):355-60. J Soc Biol. 1999. PMID: 10689617 Review. French.
-
Mechanistic aspects of mineralocorticoid receptor activation.Kidney Int. 2000 Apr;57(4):1250-5. doi: 10.1046/j.1523-1755.2000.00958.x. Kidney Int. 2000. PMID: 10760050 Review.
Cited by
-
Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation.Endocr Rev. 2012 Apr;33(2):271-99. doi: 10.1210/er.2011-1033. Epub 2012 Mar 20. Endocr Rev. 2012. PMID: 22433123 Free PMC article. Review.
-
H55N polymorphism is associated with low citrate synthase activity which regulates lipid metabolism in mouse muscle cells.PLoS One. 2017 Nov 2;12(11):e0185789. doi: 10.1371/journal.pone.0185789. eCollection 2017. PLoS One. 2017. PMID: 29095821 Free PMC article.
-
The Glucocorticoid Receptor is Required for Efficient Aldosterone-Induced Transcription by the Mineralocorticoid Receptor.bioRxiv [Preprint]. 2023 Jun 17:2023.01.26.525745. doi: 10.1101/2023.01.26.525745. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Nov 19;121(47):e2413737121. doi: 10.1073/pnas.2413737121. PMID: 36789429 Free PMC article. Updated. Preprint.
-
Genetic Profiling of Glucocorticoid (NR3C1) and Mineralocorticoid (NR3C2) Receptor Polymorphisms before Starting Therapy with Androgen Receptor Inhibitors: A Study of a Patient Who Developed Toxic Myocarditis after Enzalutamide Treatment.Biomedicines. 2022 May 29;10(6):1271. doi: 10.3390/biomedicines10061271. Biomedicines. 2022. PMID: 35740293 Free PMC article.
-
Influence of domain interactions on conformational mobility of the progesterone receptor detected by hydrogen/deuterium exchange mass spectrometry.Structure. 2014 Jul 8;22(7):961-73. doi: 10.1016/j.str.2014.04.013. Epub 2014 Jun 5. Structure. 2014. PMID: 24909783 Free PMC article.
References
-
- Lifton RP, Gharavi AG, Geller DS2001. Molecular mechanisms of human hypertension. Cell 104:545–556 - PubMed
-
- Zennaro MC, Lombès M2004. Mineralocorticoid resistance. Trends Endocrinol Metab 15:264–270 - PubMed
-
- Rogerson FM, Brennan FE, Fuller PJ2004. Mineralocorticoid receptor binding, structure and function. Mol Cell Endocrinol 217:203–212 - PubMed
-
- Fuller PJ, Young MJ2005. Mechanisms of mineralocorticoid action. Hypertension 46:1227–1235 - PubMed

