Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes
- PMID: 20685880
- PMCID: PMC2946148
- DOI: 10.1210/en.2009-1483
Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes
Abstract
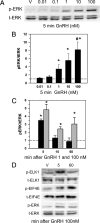
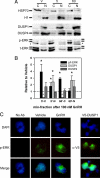
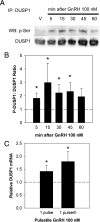
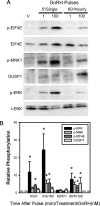
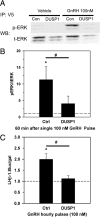
As the regulator of pituitary reproductive hormone synthesis, the hypothalamic neuropeptide GnRH is the central regulator of reproduction. A hallmark of GnRH action is the differential control of gene expression in pituitary gonadotropes through varied pulsatile stimulation. Among other signaling events, GnRH activation of the ERK family of MAPKs plays a significant role in the transcriptional regulation of the luteinizing hormone β-subunit gene and regulation of cap-dependent translation. We evaluated the ERK response to different GnRH pulse amplitudes in the gonadotrope cell line LβT2. We found that low-amplitude stimulation with GnRH invokes a rapid and transient ERK activation, whereas high-amplitude stimulation invokes a prolonged activation specifically in the cytoplasm fraction of LβT2 cells. Nuclear and cytoplasmic targets of ERK, Ets-like gene 1, and eukaryotic initiation factor 4E, respectively, are similarly activated. Feedback control of ERK activation occurs mainly through the dual-specificity protein phosphatases (DUSPs). DUSP1 is localized to the nucleus in LβT2 cells but DUSP4, another member implicated in GnRH feedback, exists in both the nucleus and cytoplasm. Manipulation of nuclear DUSP activity through overexpression or knockdown of Dusp1 modulates the ERK response to low and high GnRH pulse amplitudes and activation of the Lhb promoter. Dusp1 overexpression abolishes sustained ERK activation and inhibits Lhb promoter activity induced by high amplitude pulses. Conversely, Dusp1 knockdown enhances ERK activation by low-amplitude stimulation and increases stimulation of Lhb promoter activity. We conclude that DUSP1 feedback activity modulates ERK activation and the transcriptional response to GnRH.
Figures

Similar articles
-
Induction of dual-specificity phosphatase 1 (DUSP1) by pulsatile gonadotropin-releasing hormone stimulation: role for gonadotropin subunit expression in mouse pituitary LbetaT2 cells.Biol Reprod. 2011 May;84(5):996-1004. doi: 10.1095/biolreprod.110.088526. Epub 2011 Jan 12. Biol Reprod. 2011. PMID: 21228211
-
Induction of dual specificity phosphatase 1 (DUSP1) by gonadotropin-releasing hormone (GnRH) and the role for gonadotropin subunit gene expression in mouse pituitary gonadotroph L beta T2 cells.Biol Reprod. 2010 Feb;82(2):352-62. doi: 10.1095/biolreprod.109.080440. Epub 2009 Oct 21. Biol Reprod. 2010. PMID: 19846601
-
Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LbetaT2.Mol Endocrinol. 2004 May;18(5):1301-12. doi: 10.1210/me.2003-0478. Epub 2004 Jan 29. Mol Endocrinol. 2004. PMID: 14752057 Free PMC article.
-
Possible role of PACAP and its PAC1 receptor in the differential regulation of pituitary LHbeta- and FSHbeta-subunit gene expression by pulsatile GnRH stimulation.Biol Reprod. 2013 Feb 14;88(2):35. doi: 10.1095/biolreprod.112.105601. Print 2013 Feb. Biol Reprod. 2013. PMID: 23197164 Review.
-
GnRH signaling, the gonadotrope and endocrine control of fertility.Front Neuroendocrinol. 2010 Jul;31(3):322-40. doi: 10.1016/j.yfrne.2010.04.002. Epub 2010 May 6. Front Neuroendocrinol. 2010. PMID: 20451543 Free PMC article. Review.
Cited by
-
Induction of Stress Signaling In Vitro and Suppression of Gonadotropin Secretion by Free Fatty Acids in Female Mouse Gonadotropes.Endocrinology. 2018 Feb 1;159(2):1074-1087. doi: 10.1210/en.2017-00638. Endocrinology. 2018. PMID: 29315384 Free PMC article.
-
The RNA-Binding Protein ELAVL1 Regulates GnRH Receptor Expression and the Response to GnRH.Endocrinology. 2019 Aug 1;160(8):1999-2014. doi: 10.1210/en.2019-00203. Endocrinology. 2019. PMID: 31188427 Free PMC article.
-
GnRH Pulse Frequency Control of Fshb Gene Expression Is Mediated via ERK1/2 Regulation of ICER.Mol Endocrinol. 2016 Mar;30(3):348-60. doi: 10.1210/me.2015-1222. Epub 2016 Feb 2. Mol Endocrinol. 2016. PMID: 26835742 Free PMC article.
-
Regulation of reproduction via tight control of gonadotropin hormone levels.Mol Cell Endocrinol. 2018 Mar 5;463:116-130. doi: 10.1016/j.mce.2017.03.022. Epub 2017 Mar 22. Mol Cell Endocrinol. 2018. PMID: 28342855 Free PMC article. Review.
-
GnRH Regulates Gonadotropin Gene Expression Through NADPH/Dual Oxidase-Derived Reactive Oxygen Species.Endocrinology. 2015 Jun;156(6):2185-99. doi: 10.1210/en.2014-1709. Epub 2015 Apr 7. Endocrinology. 2015. PMID: 25849727 Free PMC article.
References
-
- Knobil E 1974 On the control of gonadotropin secretion in the rhesus monkey. Recent Prog Horm Res 30:1–46 - PubMed
-
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E 1978 Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202:631–633 - PubMed
-
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 - PubMed
-
- Rippel RH, Johnson ES, White WF 1974 Effect of consecutive injections of synthetic gonadotropin releasing hormone on LH release in the anestrous and ovariectomized ewe. J Anim Sci 39:907–914 - PubMed
-
- Naor Z 2009 Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol 30:10–29 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

