Multiple Ca2+-dependent mechanisms regulate L-type Ca2+ current in retinal amacrine cells
- PMID: 20685929
- PMCID: PMC2957451
- DOI: 10.1152/jn.00031.2010
Multiple Ca2+-dependent mechanisms regulate L-type Ca2+ current in retinal amacrine cells
Abstract
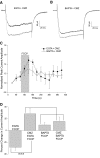
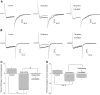
Understanding the regulation of L-type voltage-gated Ca(2+) current is an important component of elucidating the signaling capabilities of retinal amacrine cells. Here we ask how the cytosolic Ca(2+) environment and the balance of Ca(2+)-dependent effectors shape native L-type Ca(2+) channel function in these cells. To achieve this, whole cell voltage clamp recordings were made from cultured amacrine cells under conditions that address the contribution of mitochondrial Ca(2+) uptake (MCU), Ca(2+)/calmodulin (CaM)-dependent channel inactivation (CDI), protein kinase A (PKA), and Ca(2+)-induced Ca(2+) release (CICR). Under control conditions, repeated activation of the L-type channels produces a progressive enhancement of the current. Inhibition of MCU causes a reduction in the Ca(2+) current amplitude that is dependent on Ca(2+) influx as well as cytosolic Ca(2+) buffering, consistent with CDI. Including the Ca(2+) buffer bis-(o-aminophenoxy)-N,N,N',N'-tetraacetic acid (BAPTA) internally can shift the balance between enhancement and inhibition such that inhibition of MCU can enhance the current. Inhibition of PKA can remove the enhancing effect of BAPTA suggesting that cyclic AMP-dependent phosphorylation is involved. Inhibition of CaM suppresses CDI but spares the enhancement, consistent with the substantially higher sensitivity of the Ca(2+)-sensitive adenylate cyclase 1 (AC1) to Ca(2+)/CaM. Inhibition of the ryanodine receptor reduces the current amplitude, suggesting that CICR might normally amplify the activation of AC1 and stimulation of PKA activity. These experiments reveal that the amplitude of L-type Ca(2+) currents in retinal amacrine cells are both positively and negatively regulated by Ca(2+)-dependent mechanisms.
Figures

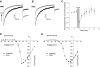

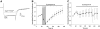





Similar articles
-
Activation of mGluR5 modulates Ca2+ currents in retinal amacrine cells from the chick.Vis Neurosci. 2004 Nov-Dec;21(6):807-16. doi: 10.1017/S0952523804216017. Vis Neurosci. 2004. PMID: 15733336
-
Mitochondrial Ca(2+) buffering regulates synaptic transmission between retinal amacrine cells.J Neurophysiol. 2002 Mar;87(3):1426-39. doi: 10.1152/jn.00627.2001. J Neurophysiol. 2002. PMID: 11877517
-
Ionic mechanisms mediating oscillatory membrane potentials in wide-field retinal amacrine cells.J Neurophysiol. 2003 Jul;90(1):431-43. doi: 10.1152/jn.00092.2003. Epub 2003 Mar 20. J Neurophysiol. 2003. PMID: 12649310
-
Nitric oxide promotes GABA release by activating a voltage-independent Ca2+ influx pathway in retinal amacrine cells.J Neurophysiol. 2017 Mar 1;117(3):1185-1199. doi: 10.1152/jn.00803.2016. Epub 2017 Jan 4. J Neurophysiol. 2017. PMID: 28053242 Free PMC article.
-
How voltage-gated ion channels alter the functional properties of ganglion and amacrine cell dendrites.Arch Ital Biol. 2002 Oct;140(4):347-59. Arch Ital Biol. 2002. PMID: 12228988 Review.
Cited by
-
Inhibition of endocytosis suppresses the nitric oxide-dependent release of Cl- in retinal amacrine cells.PLoS One. 2018 Jul 25;13(7):e0201184. doi: 10.1371/journal.pone.0201184. eCollection 2018. PLoS One. 2018. PMID: 30044876 Free PMC article.
-
Adenylate Cyclase 1 Links Calcium Signaling to CFTR-Dependent Cytosolic Chloride Elevations in Chick Amacrine Cells.Front Cell Neurosci. 2021 Aug 11;15:726605. doi: 10.3389/fncel.2021.726605. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34456687 Free PMC article.
-
A role for the cystic fibrosis transmembrane conductance regulator in the nitric oxide-dependent release of Cl- from acidic organelles in amacrine cells.J Neurophysiol. 2017 Nov 1;118(5):2842-2852. doi: 10.1152/jn.00511.2017. Epub 2017 Aug 23. J Neurophysiol. 2017. PMID: 28835528 Free PMC article.
References
-
- Baccus SA. Timing and computation in inner retinal circuitry. Annu Rev Physiol 69: 271–290, 2007 - PubMed
-
- Bean BP, Nowycky MC, Tsien RW. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. Nature 307: 371–375, 1984 - PubMed
-
- Beavo JA, Rogers NL, Crofford OB, Hardman JG, Sutherland EW, Newman EV. Effects of xanthine derivatives on lipolysis and on adenosine 3′,5′-monophosphate phosphodiesterase activity. Mol Pharmacol 6: 597–603, 1970 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

