Mitochondrial SIRT3 and heart disease
- PMID: 20685942
- PMCID: PMC2952535
- DOI: 10.1093/cvr/cvq250
Mitochondrial SIRT3 and heart disease
Abstract
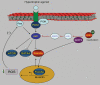
Sirtuins are emerging as key regulators of many cellular functions including metabolism, cell growth, apoptosis, and genetic control of ageing. In mammals there are seven sirtuin analogues, SIRT1 to SIRT7. Among them SIRT3 is unique because this is the only analogue whose increased expression has been found to be associated with extended lifespan of humans. SIRT3 levels have been shown to be elevated by exercise and calorie restriction. Although the role of SIRT3 in cell biology is only beginning to be understood, initial studies have shown that SIRT3 plays a major role in free fatty acid oxidation and maintenance of cellular ATP levels. In the heart SIRT3 has been found to block development of cardiac hypertrophy and protect cardiomyocytes from oxidative stress-mediated cell death. Similarly, SIRT3 has been reported to have tumour-suppressive characteristics. In this article, we review the known effects of SIRT3 in different tissues and relate them to the protection of cardiomyocytes under stress.
Figures
References
-
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi:10.1038/nrm1616. - DOI - PubMed
-
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi:10.1016/j.molcel.2007.07.032. - DOI - PubMed
-
- Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi:10.1038/nature07349. - DOI - PMC - PubMed
-
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi:10.1016/S0092-8674(04)00126-6. - DOI - PubMed
-
- Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. ProcNatl Acad Sci. 2007;104:12861–12866. doi:10.1073/pnas.0702509104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

