Ang2/fat-free is a conserved subunit of the Golgi-associated retrograde protein complex
- PMID: 20685960
- PMCID: PMC2947474
- DOI: 10.1091/mbc.E10-05-0392
Ang2/fat-free is a conserved subunit of the Golgi-associated retrograde protein complex
Abstract
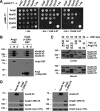
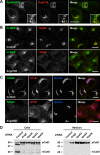
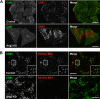
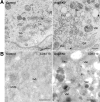
The Golgi-associated retrograde protein (GARP) complex mediates tethering and fusion of endosome-derived transport carriers to the trans-Golgi network (TGN). In the yeast Saccharomyces cerevisiae, GARP comprises four subunits named Vps51p, Vps52p, Vps53p, and Vps54p. Orthologues of the GARP subunits, except for Vps51p, have been identified in all other eukaryotes. A yeast two-hybrid screen of a human cDNA library yielded a phylogenetically conserved protein, Ang2/Fat-free, which interacts with human Vps52, Vps53 and Vps54. Human Ang2 is larger than yeast Vps51p, but exhibits significant homology in an N-terminal coiled-coil region that mediates assembly with other GARP subunits. Biochemical analyses show that human Ang2, Vps52, Vps53 and Vps54 form an obligatory 1:1:1:1 complex that strongly interacts with the regulatory Habc domain of the TGN SNARE, Syntaxin 6. Depletion of Ang2 or the GARP subunits similarly impairs protein retrieval to the TGN, lysosomal enzyme sorting, endosomal cholesterol traffic¤ and autophagy. These findings indicate that Ang2 is the missing component of the GARP complex in most eukaryotes.
Figures


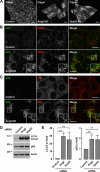
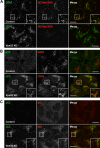
References
-
- Ballabio A. Disease pathogenesis explained by basic science: lysosomal storage diseases as autophagocytic disorders. Int. J. Clin. Pharmacol. Ther. 2009;47(Suppl 1):S34–S38. - PubMed
-
- Cai H., Reinisch K., Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell. 2007;12:671–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

