Vestigial is required during late-stage muscle differentiation in Drosophila melanogaster embryos
- PMID: 20685961
- PMCID: PMC2947467
- DOI: 10.1091/mbc.E10-04-0364
Vestigial is required during late-stage muscle differentiation in Drosophila melanogaster embryos
Abstract
The somatic muscles of Drosophila develop in a complex pattern that is repeated in each embryonic hemi-segment. During early development, progenitor cells fuse to form a syncytial muscle, which further differentiates via expression of muscle-specific factors that induce specific responses to external signals to regulate late-stage processes such as migration and attachment. Initial communication between somatic muscles and the epidermal tendon cells is critical for both of these processes. However, later establishment of attachments between longitudinal muscles at the segmental borders is largely independent of the muscle-epidermal attachment signals, and relatively little is known about how this event is regulated. Using a combination of null mutations and a truncated version of Sd that binds Vg but not DNA, we show that Vestigial (Vg) is required in ventral longitudinal muscles to induce formation of stable intermuscular attachments. In several muscles, this activity may be independent of Sd. Furthermore, the cell-specific differentiation events induced by Vg in two cells fated to form attachments are coordinated by Drosophila epidermal growth factor signaling. Thus, Vg is a key factor to induce specific changes in ventral longitudinal muscles 1-4 identity and is required for these cells to be competent to form stable intermuscular attachments with each other.
Figures

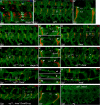

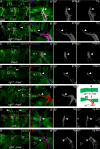
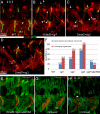
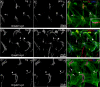
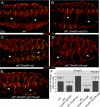
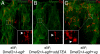
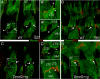
Similar articles
-
Moleskin is essential for the formation of the myotendinous junction in Drosophila.Dev Biol. 2011 Nov 15;359(2):176-89. doi: 10.1016/j.ydbio.2011.08.028. Epub 2011 Sep 9. Dev Biol. 2011. PMID: 21925492 Free PMC article.
-
WNT5 interacts with the Ryk receptors doughnut and derailed to mediate muscle attachment site selection in Drosophila melanogaster.PLoS One. 2012;7(3):e32297. doi: 10.1371/journal.pone.0032297. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403643 Free PMC article.
-
"Importin" signaling roles for import proteins: the function of Drosophila importin-7 (DIM-7) in muscle-tendon signaling.Cell Adh Migr. 2012 Jan-Feb;6(1):4-12. doi: 10.4161/cam.19774. Cell Adh Migr. 2012. PMID: 22647935 Free PMC article.
-
Singling out Drosophila tendon cells: a dialogue between two distinct cell types.Trends Genet. 1999 Nov;15(11):448-53. doi: 10.1016/s0168-9525(99)01862-4. Trends Genet. 1999. PMID: 10529807 Review.
-
Building functional units of movement-generation and movement-sensation in the embryo.Int J Dev Biol. 2017;61(3-4-5):171-178. doi: 10.1387/ijdb.160279as. Int J Dev Biol. 2017. PMID: 28621415 Review.
Cited by
-
Genetic Control of Muscle Diversification and Homeostasis: Insights from Drosophila.Cells. 2020 Jun 25;9(6):1543. doi: 10.3390/cells9061543. Cells. 2020. PMID: 32630420 Free PMC article. Review.
-
Intrinsic control of muscle attachment sites matching.Elife. 2020 Jul 24;9:e57547. doi: 10.7554/eLife.57547. Elife. 2020. PMID: 32706334 Free PMC article.
-
From vestigial to vestigial-like: the Drosophila gene that has taken wing.Dev Genes Evol. 2016 Jul;226(4):297-315. doi: 10.1007/s00427-016-0546-3. Epub 2016 Apr 26. Dev Genes Evol. 2016. PMID: 27116603 Review.
-
Drosophila araucan and caupolican integrate intrinsic and signalling inputs for the acquisition by muscle progenitors of the lateral transverse fate.PLoS Genet. 2011 Jul;7(7):e1002186. doi: 10.1371/journal.pgen.1002186. Epub 2011 Jul 21. PLoS Genet. 2011. PMID: 21811416 Free PMC article.
-
Wnt-mediated repression via bipartite DNA recognition by TCF in the Drosophila hematopoietic system.PLoS Genet. 2014 Aug 21;10(8):e1004509. doi: 10.1371/journal.pgen.1004509. eCollection 2014 Aug. PLoS Genet. 2014. PMID: 25144371 Free PMC article.
References
-
- Bate M. The Development of Drosophila melanogaster, II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. The mesoderm and its derivatives; pp. 1013–1090.
-
- Baylies M. K., Bate M., Ruiz Gomez M. Myogenesis: a view from Drosophila. Cell. 1998;93:921–927. - PubMed
-
- Becker S., Pasca G., Strumpf D., Min L., Volk T. Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development. 1997;124:2615–2622. - PubMed
-
- Bernard F., Dutriaux A., Silber J., Lalouette A. Notch pathway repression by vestigial is required to promote indirect flight muscle differentiation in Drosophila melanogaster. Dev. Biol. 2006;295:164–177. - PubMed
-
- Bernard F., Lalouette A., Gullaud M., Jeantet A. Y., Cossard R., Zider A., Ferveur J. F., Silber J. Control of apterous by vestigial drives indirect flight muscle development in Drosophila. Dev. Biol. 2003;260:391–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

