Different localizations and cellular behaviors of leiomodin and tropomodulin in mature cardiomyocyte sarcomeres
- PMID: 20685966
- PMCID: PMC2947471
- DOI: 10.1091/mbc.E10-02-0109
Different localizations and cellular behaviors of leiomodin and tropomodulin in mature cardiomyocyte sarcomeres
Abstract
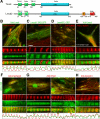
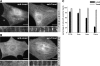
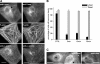
Leiomodin (Lmod) is a muscle-specific F-actin-nucleating protein that is related to the F-actin pointed-end-capping protein tropomodulin (Tmod). However, Lmod contains a unique ∼150-residue C-terminal extension that is required for its strong nucleating activity. Overexpression or depletion of Lmod compromises sarcomere organization, but the mechanism by which Lmod contributes to myofibril assembly is not well understood. We show that Tmod and Lmod localize through fundamentally different mechanisms to the pointed ends of two distinct subsets of actin filaments in myofibrils. Tmod localizes to two narrow bands immediately adjacent to M-lines, whereas Lmod displays dynamic localization to two broader bands, which are generally more separated from M-lines. Lmod's localization and F-actin nucleation activity are enhanced by interaction with tropomyosin. Unlike Tmod, the myofibril localization of Lmod depends on sustained muscle contraction and actin polymerization. We further show that Lmod expression correlates with the maturation of myofibrils in cultured cardiomyocytes and that it associates with sarcomeres only in differentiated myofibrils. Collectively, the data suggest that Lmod contributes to the final organization and maintenance of sarcomere architecture by promoting tropomyosin-dependent actin filament nucleation.
Figures


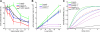
Similar articles
-
Leiomodin is an actin filament nucleator in muscle cells.Science. 2008 Apr 11;320(5873):239-43. doi: 10.1126/science.1155313. Science. 2008. PMID: 18403713 Free PMC article.
-
The N-terminal tropomyosin- and actin-binding sites are important for leiomodin 2's function.Mol Biol Cell. 2016 Aug 15;27(16):2565-75. doi: 10.1091/mbc.E16-03-0200. Epub 2016 Jun 15. Mol Biol Cell. 2016. PMID: 27307584 Free PMC article.
-
Interaction of cardiac leiomodin with the native cardiac thin filament.PLoS Biol. 2025 Jan 30;23(1):e3003027. doi: 10.1371/journal.pbio.3003027. eCollection 2025 Jan. PLoS Biol. 2025. PMID: 39883708 Free PMC article.
-
Role of intrinsic disorder in muscle sarcomeres.Prog Mol Biol Transl Sci. 2019;166:311-340. doi: 10.1016/bs.pmbts.2019.03.014. Epub 2019 Apr 13. Prog Mol Biol Transl Sci. 2019. PMID: 31521234 Free PMC article. Review.
-
Tropomodulin capping of actin filaments in striated muscle development and physiology.J Biomed Biotechnol. 2011;2011:103069. doi: 10.1155/2011/103069. Epub 2011 Oct 17. J Biomed Biotechnol. 2011. PMID: 22013379 Free PMC article. Review.
Cited by
-
Tropomodulins: pointed-end capping proteins that regulate actin filament architecture in diverse cell types.Cytoskeleton (Hoboken). 2012 Jun;69(6):337-70. doi: 10.1002/cm.21031. Epub 2012 May 4. Cytoskeleton (Hoboken). 2012. PMID: 22488942 Free PMC article. Review.
-
RNA sequencing to study gene expression and SNP variations associated with growth in zebrafish fed a plant protein-based diet.Mar Biotechnol (NY). 2015 Jun;17(3):353-63. doi: 10.1007/s10126-015-9624-1. Epub 2015 Feb 22. Mar Biotechnol (NY). 2015. PMID: 25702041
-
DAAM is required for thin filament formation and Sarcomerogenesis during muscle development in Drosophila.PLoS Genet. 2014 Feb 27;10(2):e1004166. doi: 10.1371/journal.pgen.1004166. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24586196 Free PMC article.
-
Tropomodulin 1 directly controls thin filament length in both wild-type and tropomodulin 4-deficient skeletal muscle.Development. 2015 Dec 15;142(24):4351-62. doi: 10.1242/dev.129171. Epub 2015 Nov 19. Development. 2015. PMID: 26586224 Free PMC article.
-
How Leiomodin and Tropomodulin use a common fold for different actin assembly functions.Nat Commun. 2015 Sep 15;6:8314. doi: 10.1038/ncomms9314. Nat Commun. 2015. PMID: 26370058 Free PMC article.
References
-
- Agarkova I., Perriard J. C. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. - PubMed
-
- Chhabra E. S., Higgs H. N. The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 2007;9:1110–1121. - PubMed
-
- Clark K. A., McElhinny A. S., Beckerle M. C., Gregorio C. C. Striated muscle cytoarchitecture: an intricate web of form and function. Annu. Rev. Cell Dev. Biol. 2002;18:637–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

