Lipoprotein particles cross the blood-brain barrier in Drosophila
- PMID: 20685986
- PMCID: PMC6634680
- DOI: 10.1523/JNEUROSCI.5943-09.2010
Lipoprotein particles cross the blood-brain barrier in Drosophila
Abstract
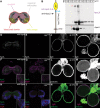
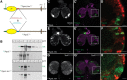
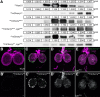
The blood-brain barrier (BBB) regulates passage of nutrients and signaling molecules from the circulation into the brain. Whether lipoproteins cross the BBB in vivo has been controversial, and no clear requirement for circulating lipoproteins in brain development has been shown. We address these issues in Drosophila, which has an functionally conserved BBB, and lipoproteins that resemble those of vertebrates. We show that the Drosophila lipoprotein lipophorin exists in two isoforms. Both isoforms cross the BBB, but accumulate on distinct subsets of cells within the brain. In addition to acting as a lipid carrier, lipophorin carries both sterol-linked and GPI-linked proteins into the circulation and transports them across the BBB. Finally, lipophorin promotes neuroblast proliferation by a mechanism that does not depend on delivery of dietary lipids. Transport of lipophorin and its cargo across the BBB represents a novel mechanism by which peripherally synthesized proteins might enter the brain and influence its development. Furthermore, lipid-linkage may be an efficient method to transport therapeutic molecules across the BBB.
Figures

References
-
- Balazs Z, Panzenboeck U, Hammer A, Sovic A, Quehenberger O, Malle E, Sattler W. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J Neurochem. 2004;89:939–950. - PubMed
-
- Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
