Selection of prime actor in humans during bimanual object manipulation
- PMID: 20685987
- PMCID: PMC6634672
- DOI: 10.1523/JNEUROSCI.1624-10.2010
Selection of prime actor in humans during bimanual object manipulation
Abstract
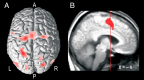
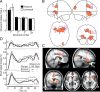
In bimanual object manipulation tasks, people flexibly assign one hand as a prime actor while the other assists. Little is known, however, about the neural mechanisms deciding the role assignment. We addressed this issue in a task in which participants moved a cursor to hit targets on a screen by applying precisely coupled symmetrical opposing linear and twist forces on a tool held freely between the hands. In trials presented in an unpredictable order, the action of either the left or the right hand was spatially congruent with the cursor movements, which automatically rendered the left or right hand the dominant actor, respectively. Functional magnetic resonance imaging indicated that the hand-selection process engaged prefrontal cortical areas belonging to an executive control network presumed critical for judgment and decision-making and to a salience network attributed to evaluation of utility of actions. Task initiation, which involved switching between task sets, had a superordinate role with reference to hand selection. Behavioral and brain imaging data indicated that participants initially expressed two competing action representations, matching either mapping rule, before selecting the appropriate one based on the consequences of the initial manual actions. We conclude that implicit processes engaging the prefrontal cortex reconcile selections among action representations that compete for the establishment of a dominant actor in bimanual object manipulation tasks. The representation selected is the one that optimizes performance by relying on the superior capacity of the brain to process spatial congruent, as opposed to noncongruent, mappings between manual actions and desired movement goals.
Figures




Similar articles
-
Zones of bimanual and unimanual preference within human primary sensorimotor cortex during object manipulation.Neuroimage. 2007;36 Suppl 2:T2-T15. doi: 10.1016/j.neuroimage.2007.03.042. Epub 2007 Mar 31. Neuroimage. 2007. PMID: 17499166
-
How a lateralized brain supports symmetrical bimanual tasks.PLoS Biol. 2006 Jun;4(6):e158. doi: 10.1371/journal.pbio.0040158. Epub 2006 May 9. PLoS Biol. 2006. PMID: 16669700 Free PMC article.
-
Can left-handedness be switched? Insights from an early switch of handwriting.J Neurosci. 2007 Jul 18;27(29):7847-53. doi: 10.1523/JNEUROSCI.1299-07.2007. J Neurosci. 2007. PMID: 17634378 Free PMC article.
-
Resource-demanding versus cost-effective bimanual interaction in the brain.Exp Brain Res. 2010 Jun;203(2):407-18. doi: 10.1007/s00221-010-2244-0. Epub 2010 Apr 24. Exp Brain Res. 2010. PMID: 20419370
-
Reward-dependent learning in neuronal networks for planning and decision making.Prog Brain Res. 2000;126:217-29. doi: 10.1016/S0079-6123(00)26016-0. Prog Brain Res. 2000. PMID: 11105649 Review.
Cited by
-
Hand pose selection in a bimanual fine-manipulation task.J Neurophysiol. 2021 Jul 1;126(1):195-212. doi: 10.1152/jn.00635.2020. Epub 2021 Jun 9. J Neurophysiol. 2021. PMID: 34107225 Free PMC article.
-
Human posterior parietal cortex mediates hand-specific planning.Neuroimage. 2015 Jul 1;114:226-38. doi: 10.1016/j.neuroimage.2015.03.058. Epub 2015 Apr 2. Neuroimage. 2015. PMID: 25842294 Free PMC article.
-
Hand use for grasping in a bimanual task: evidence for different roles?Exp Brain Res. 2013 Feb;224(3):455-67. doi: 10.1007/s00221-012-3325-z. Epub 2012 Nov 18. Exp Brain Res. 2013. PMID: 23161156
References
-
- Bunge SA. How we use rules to select actions: a review of evidence from cognitive neuroscience. Cogn Affect Behav Neurosci. 2004;4:564–579. - PubMed
-
- Burnod Y, Baraduc P, Battaglia-Mayer A, Guigon E, Koechlin E, Ferraina S, Lacquaniti F, Caminiti R. Parieto-frontal coding of reaching: an integrated framework. Exp Brain Res. 1999;129:325–346. - PubMed
-
- Cao J. The size of the connected components of excursion sets of χ2, t and F fields. Adv Appl Probab. 1999;31:579–595.
-
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45:801–814. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
