Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells
- PMID: 20685991
- PMCID: PMC2935844
- DOI: 10.1523/JNEUROSCI.4721-09.2010
Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells
Abstract
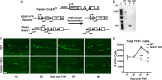
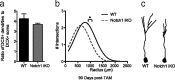
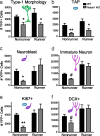
Notch1 regulates neural stem cell (NSC) number during development, but its role in adult neurogenesis is unclear. We generated nestin-CreER(T2)/R26R-YFP/Notch1(loxP/loxP) [Notch1inducible knock-out (iKO)] mice to allow tamoxifen (TAM)-inducible elimination of Notch1 and concomitant expression of yellow fluorescent protein (YFP) in nestin-expressing Type-1 NSCs and their progeny in the adult hippocampal subgranular zone (SGZ). Consistent with previous research, YFP+ cells in all stages of neurogenesis were evident in the subgranular zone (SGZ) of wild-type (WT) mice (nestin-CreER(T2)/R26R-YFP/Notch1(w/w)) after tamoxifen (post-TAM), producing adult-generated YFP+ dentate gyrus neurons. Compared with WT littermates, Notch1 iKO mice had similar numbers of total SGZ YFP+ cells 13 and 30 d post-TAM but had significantly fewer SGZ YFP+ cells 60 and 90 d post-TAM. Significantly fewer YFP+ Type-1 NSCs and transiently amplifying progenitors (TAPs) resulted in generation of fewer YFP+ granule neurons in Notch1 iKO mice. Strikingly, 30 d of running rescued this deficit, as the total YFP+ cell number in Notch iKO mice was equivalent to WT levels. This was even more notable given the persistent deficits in the Type-1 NSC and TAP reservoirs. Our data show that Notch1 signaling is required to maintain a reservoir of undifferentiated cells and ensure continuity of adult hippocampal neurogenesis, but that alternative Notch- and Type-1 NSC-independent pathways compensate in response to physical activity. These data shed light on the complex relationship between Type-1 NSCs, adult neurogenesis, the neurogenic niche, and environmental stimuli.
Figures




References
-
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. - PubMed
-
- Annenkov A. The insulin-like growth factor (IGF) receptor type 1 (IGF1R) as an essential component of the signalling network regulating neurogenesis. Mol Neurobiol. 2009;40:195–215. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Bednarczyk MR, Aumont A, Décary S, Bergeron R, Fernandes KJ. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus. 2009;19:913–927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
