Temporal discounting of reward and the cost of time in motor control
- PMID: 20685993
- PMCID: PMC2926660
- DOI: 10.1523/JNEUROSCI.1343-10.2010
Temporal discounting of reward and the cost of time in motor control
Abstract
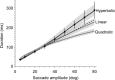
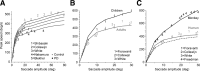
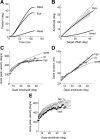
Why do movements take a characteristic amount of time, and why do diseases that affect the reward system alter control of movements? Suppose that the purpose of any movement is to position our body in a more rewarding state. People and other animals discount future reward as a hyperbolic function of time. Here, we show that across populations of people and monkeys there is a correlation between discounting of reward and control of movements. We consider saccadic eye movements and hypothesize that duration of a movement is equivalent to a delay of reward. The hyperbolic cost of this delay not only accounts for kinematics of saccades in adults, it also accounts for the faster saccades of children, who temporally discount reward more steeply. Our theory explains why saccade velocities increase when reward is elevated, and why disorders in the encoding of reward, for example in Parkinson's disease and schizophrenia, produce changes in saccade. We show that delay of reward elevates the cost of saccades, reducing velocities. Finally, we consider coordinated movements that include motion of eyes and head and find that their kinematics is also consistent with a hyperbolic, reward-dependent cost of time. Therefore, each voluntary movement carries a cost because its duration delays acquisition of reward. The cost depends on the value that the brain assigns to stimuli, and the rate at which it discounts this value in time. The motor commands that move our eyes reflect this cost of time.
Figures


References
-
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Processes. 2003;64:345–354. - PubMed
-
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–454. - PubMed
-
- Bizzi E. The coordination of eye-head movements. Sci Am. 1974;231:100–106. - PubMed
-
- Blekher T, Siemers E, Abel LA, Yee RD. Eye movements in Parkinson's disease: before and after pallidotomy. Invest Ophthalmol Vis Sci. 2000;41:2177–2183. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
