LIM-homeobox gene Lhx5 is required for normal development of Cajal-Retzius cells
- PMID: 20685998
- PMCID: PMC2927820
- DOI: 10.1523/JNEUROSCI.5563-09.2010
LIM-homeobox gene Lhx5 is required for normal development of Cajal-Retzius cells
Abstract
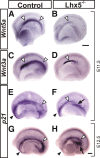
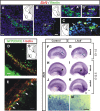
Cajal-Retzius (C-R) cells play important roles in the lamination of the mammalian cortex via reelin secretion. The genetic mechanisms underlying the development of these neurons have just begun to be unraveled. Here, we show that two closely related LIM-homeobox genes Lhx1 and Lhx5 are expressed in reelin+ cells in various regions in the mouse telencephalon at or adjacent to sites where the C-R cells are generated, including the cortical hem, the mantle region of the septal/retrobulbar area, and the ventral pallium. Whereas Lhx5 is expressed in all of these reelin-expressing domains, Lhx1 is preferentially expressed in the septal area and in a continuous domain spanning from lateral olfactory region to caudomedial territories. Genetic ablation of Lhx5 results in decreased reelin+ and p73+ cells in the neocortical anlage, in the cortical hem, and in the septal, olfactory, and caudomedial telencephalic regions. The overall reduction in number of C-R cells in Lhx5 mutants is accompanied by formation of ectopic reelin+ cell clusters at the caudal telencephalon. Based on differential expression of molecular markers and by fluorescent cell tracing in cultured embryos, we located the origin of reelin+ ectopic cell clusters at the caudomedial telencephalic region. We also confirmed the existence of a normal migration stream of reelin+ cells from the caudomedial area to telencephalic olfactory territories in wild-type embryos. These results reveal a complex role for Lhx5 in regulating the development and normal distribution of C-R cells in the developing forebrain.
Figures







References
-
- Abellan A, Menuet A, Dehay C, Medina L, Retaux S. Differential expression of LIM-homeodomain factors in Cajal-Retzius cells of primates, rodents, and birds. Cereb Cortex. 2010;20:1788–1798. - PubMed
-
- Bielle F, Griveau A, Narboux-Nême N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
