A single-amino-acid substitution in herpes simplex virus 1 envelope glycoprotein B at a site required for binding to the paired immunoglobulin-like type 2 receptor alpha (PILRalpha) abrogates PILRalpha-dependent viral entry and reduces pathogenesis
- PMID: 20686018
- PMCID: PMC2950593
- DOI: 10.1128/JVI.01166-10
A single-amino-acid substitution in herpes simplex virus 1 envelope glycoprotein B at a site required for binding to the paired immunoglobulin-like type 2 receptor alpha (PILRalpha) abrogates PILRalpha-dependent viral entry and reduces pathogenesis
Abstract
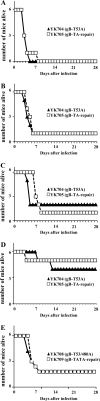
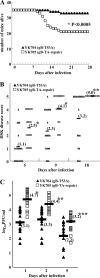
Paired immunoglobulin-like type 2 receptor α (PILRα) is a herpes simplex virus 1 (HSV-1) entry receptor that associates with O-glycans on HSV-1 envelope glycoprotein B (gB). Two threonine residues (Thr-53 and Thr-480) in gB, which are required for the addition of the principal gB O-glycans, are essential for binding to soluble PILRα. However, the role of the two threonines in PILRα-dependent viral entry remains to be elucidated. Therefore, we constructed a recombinant HSV-1 carrying an alanine replacement of gB Thr-53 alone (gB-T53A) or of both gB Thr-53 and Thr-480 (gB-T53/480A) and demonstrated that these mutations abrogated viral entry in CHO cells expressing PILRα. In contrast, the mutations had no effect on viral entry in CHO cells expressing known host cell receptors for HSV-1 gD, viral entry in HL60 cells expressing myelin-associated glycoprotein (MAG) (another HSV-1 gB receptor), viral attachment to heparan sulfate, and viral replication in PILRα-negative cells. These results support the hypothesis that gB Thr-53 and Thr-480 as well as gB O-glycosylation, probably at these sites, are critical for PILRα-dependent viral entry. Interestingly, following corneal inoculation in mice, the gB-T53A and gB-T53/480A mutations significantly reduced viral replication in the cornea, the development of herpes stroma keratitis, and neuroinvasiveness. The abilities of HSV-1 to enter cells in a PILRα-dependent manner and to acquire specific carbohydrates on gB are therefore linked to an increase in viral replication and virulence in the experimental murine model.
Figures




Similar articles
-
Differential effects on cell fusion activity of mutations in herpes simplex virus 1 glycoprotein B (gB) dependent on whether a gD receptor or a gB receptor is overexpressed.J Virol. 2009 Aug;83(15):7384-90. doi: 10.1128/JVI.00087-09. Epub 2009 May 20. J Virol. 2009. PMID: 19457990 Free PMC article.
-
The amino terminus of herpes simplex virus 1 glycoprotein K is required for virion entry via the paired immunoglobulin-like type-2 receptor alpha.J Virol. 2013 Mar;87(6):3305-13. doi: 10.1128/JVI.02982-12. Epub 2013 Jan 9. J Virol. 2013. PMID: 23302878 Free PMC article.
-
Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1.J Virol. 2003 Sep;77(17):9221-31. doi: 10.1128/jvi.77.17.9221-9231.2003. J Virol. 2003. PMID: 12915538 Free PMC article.
-
HSV-1 infection through inhibitory receptor, PILRalpha.Uirusu. 2008 Jun;58(1):27-36. doi: 10.2222/jsv.58.27. Uirusu. 2008. PMID: 19122386 Review.
-
Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion.Viruses. 2021 Sep 16;13(9):1849. doi: 10.3390/v13091849. Viruses. 2021. PMID: 34578430 Free PMC article. Review.
Cited by
-
Identification and phylogenetic analysis of herpes simplex virus-1 from clinical isolates in India.Access Microbiol. 2019 Jul 26;1(6):e000047. doi: 10.1099/acmi.0.000047. eCollection 2019. Access Microbiol. 2019. PMID: 32974534 Free PMC article.
-
Global aspects of viral glycosylation.Glycobiology. 2018 Jul 1;28(7):443-467. doi: 10.1093/glycob/cwy021. Glycobiology. 2018. PMID: 29579213 Free PMC article.
-
Structure of the Kaposi's sarcoma-associated herpesvirus gB in post-fusion conformation.J Virol. 2025 Feb 25;99(2):e0153324. doi: 10.1128/jvi.01533-24. Epub 2025 Jan 17. J Virol. 2025. PMID: 39818969 Free PMC article.
-
Entry of Alphaherpesviruses.Curr Issues Mol Biol. 2021;41:63-124. doi: 10.21775/cimb.041.063. Epub 2020 Aug 7. Curr Issues Mol Biol. 2021. PMID: 32764159 Free PMC article. Review.
-
Site Specific N- and O-glycosylation mapping of the Spike Proteins of SARS-CoV-2 Variants of Concern.Res Sq [Preprint]. 2022 Nov 16:rs.3.rs-2188138. doi: 10.21203/rs.3.rs-2188138/v1. Res Sq. 2022. Update in: Sci Rep. 2023 Jun 21;13(1):10053. doi: 10.1038/s41598-023-33088-0. PMID: 36415454 Free PMC article. Updated. Preprint.
References
-
- Bosnjak, L., M. Miranda-Saksena, D. M. Koelle, R. A. Boadle, C. A. Jones, and A. L. Cunningham. 2005. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J. Immunol. 174:2220-2227. - PubMed
-
- Carr, D. J., P. Harle, and B. M. Gebhardt. 2001. The immune response to ocular herpes simplex virus type 1 infection. Exp. Biol. Med. (Maywood) 226:353-366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

